As filed with the Securities and Exchange Commission on June 30, 2025.
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
_____________________________________
FORM
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
_____________________________________
(Exact Name of Registrant as Specified in its Constitution)
Not Applicable
(Translation of Registrant name into English)
_____________________________________
| | 2834 | N/A | ||
| (State or Other Jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer |
100 Albert Road
Australia
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
_____________________________________
Tel:
(Name, address, including zip code, and telephone number, including area code, of agent for service)
_____________________________________
With copies to:
|
Richard I. Anslow, Esq. |
Clayton E. Parker, Esq. |
_____________________________________
Approximate date of commencement of proposed sale to the public: As soon as practicable after effectiveness of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933.
Emerging growth company
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised accounting standards† provided to Section 7(a)(2)(B) of the Securities Act.
____________
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
|
PRELIMINARY PROSPECTUS |
SUBJECT TO COMPLETION, DATED JUNE 30, 2025 |
Up to 4,000,000 Ordinary Shares
Gelteq Limited
This prospectus relates to the resale, from time to time, of up to 4,000,000 of our ordinary shares, no par value (the “Ordinary Shares”), by the selling shareholder, Lincoln Park Capital Fund, LLC (“Lincoln Park”, or the “selling shareholder”).
The Ordinary Shares being offered by the selling shareholder have been or may be issued pursuant to that certain purchase agreement between us and Lincoln Park, dated as of March 13, 2025 (the “Purchase Agreement”). See “The Lincoln Park Transaction” for a description of the Purchase Agreement and “Selling Shareholder” for additional information regarding Lincoln Park. The prices at which Lincoln Park may sell the shares will be determined by the prevailing market price for the shares or in negotiated transactions.
We may receive gross proceeds of up to $12,000,000 from the sale of our Ordinary Shares (“Purchase Shares”) to Lincoln Park under the Purchase Agreement, from time to time, in our discretion after the date of the registration statement of which this prospectus is a part is declared effective and after satisfaction of other conditions in the Purchase Agreement. We are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of the shares by the selling shareholder.
Lincoln Park may sell the Ordinary Shares described in this prospectus in a number of different ways and at varying prices. The price that Lincoln Park will pay for the shares to be resold pursuant to this prospectus will depend upon the timing of sales and will fluctuate based on the trading price of our Ordinary Shares. Lincoln Park is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended (the “Securities Act”).
The purchase price for the Purchase Shares will be based upon formulas set forth in the Purchase Agreement depending on the type of purchase notice we submit to Lincoln Park from time to time. We will pay the expenses incurred in registering the Ordinary Shares, including legal and accounting fees. See “Plan of Distribution” on page 80 for more information about how Lincoln Park may sell the Ordinary Shares being registered pursuant to this prospectus.
We previously closed our initial public offering (“IPO”) on October 30, 2024. Our Ordinary Shares are listed on the Nasdaq Capital Market (“Nasdaq”) under the symbol “GELS.” On June 27, 2025, the closing price of our Ordinary Shares was $1.81 per share.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our Ordinary Shares involves a high degree of risk. See “Risk Factors” beginning on page 8 for a discussion of information that should be considered in connection with an investment in our Ordinary Shares.
Neither the Securities and Exchange Commission nor any other state securities commission has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2025.
TABLE OF CONTENTS
|
Page |
||
|
ii |
||
|
iii |
||
|
iv |
||
|
vi |
||
|
1 |
||
|
5 |
||
|
8 |
||
|
16 |
||
|
17 |
||
|
22 |
||
|
23 |
||
|
MANAGEMENT DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
24 |
|
|
43 |
||
|
69 |
||
|
75 |
||
|
76 |
||
|
77 |
||
|
78 |
||
|
80 |
||
|
82 |
||
|
83 |
||
|
83 |
||
|
83 |
||
|
84 |
You should rely only on the information contained or incorporated by reference in this prospectus or in any related free-writing prospectus. Neither we nor the selling shareholder has authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses prepared by us or on our behalf or to which we have referred you. We take no responsibility for and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the Ordinary Shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. We are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted or where the person making the offer or sale is not qualified to do so or to any person to whom it is not permitted to make such offer or sale. We have not taken any action to permit a public offering of the Ordinary Shares outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of the Ordinary Shares and the distribution of the prospectus outside the United States. The information contained in this prospectus is current only as of the date on the front cover of the prospectus. Our business, financial condition, results of operations and prospects may have changed since that date.
We are incorporated as an Australian public limited company limited to shares under the laws of Australia pursuant to our constitution, and a majority of our outstanding securities are owned by non-U.S. residents. Under the rules of the U.S. Securities and Exchange Commission, or SEC, we are currently eligible for treatment as a “foreign private issuer,” or FPI. As an FPI, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as domestic registrants whose securities are registered under the Securities Exchange Act of 1934, as amended, or the Exchange Act.
Until , 2025 (the 25th day after the date of this prospectus), all dealers that buy, sell or trade Ordinary Shares, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the obligation of dealers to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
i
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form F-1 filed with the SEC by Gelteq Limited, an Australian public limited company limited to shares pursuant to its Constitution. This prospectus includes important information about us, the Ordinary Shares and other information you should know before investing in the Ordinary Shares. This prospectus does not contain all of the information provided in the registration statement that we filed with the SEC. You should read this prospectus together with the additional information about us described in the section below entitled “Where You Can Find Additional Information.”
For investors outside of the United States of America (the “United States” or the “U.S.”): Neither we nor the selling shareholder have done anything to permit the conduct of this offering or the possession or distribution of this prospectus in any jurisdiction, other than the United States, where action for any such purpose would be required. Persons outside of the United States who come into possession of this prospectus must inform themselves about, and observe, any restrictions relating to the conduct of this offering and the possession and distribution of this prospectus that apply in the jurisdictions outside of the United States relevant to their circumstances.
Unless otherwise indicated or the context otherwise requires, all references in this prospectus to “Gelteq Limited,” “Gelteq,” “our company,” “the company” “we,” “us,” and “our” refer to Gelteq Limited and its consolidated subsidiaries.
Unless otherwise indicated or the context otherwise requires, all references in this prospectus to legislation are to federal, state and local legislation of the United States.
Unless otherwise indicated, references to a particular “fiscal year” are to our fiscal year ended June 30th of that year. Our fiscal quarters end on September 30th, December 31st, March 31st and June 30th of each fiscal year (for which purpose June 30th is also our fiscal year end). References to a year other than a “Fiscal” or “fiscal year” are to the calendar year ended December 31.
In this prospectus, all references to “Ordinary Shares” mean our Ordinary Shares, no par value.
In this prospectus, all references to the “Constitution” are to our new constitution as an Australian public company which became effective on May 26, 2022. Prior to May 26, 2022, the Company was a private company named Gelteq Pty Ltd, and on conversion to a public company on such date, the name of the Company changed to Gelteq Ltd. However, there has been no financial restructuring resulting upon the conversion of Gelteq Pty Ltd into a public company and Gelteq Limited is the same company as Gelteq Pty Ltd for financial, tax and other purposes.
This prospectus and the information incorporated herein by reference contain market data, industry statistics and other data that have been obtained from, or compiled from, information made available by third parties. We have not independently verified their data.
In this registration statement, any reference to any provision of any legislation shall include any amendment, modification, re-enactment or extension thereof. Words importing the singular shall include the plural and vice versa, and words importing the masculine gender shall include the feminine or neutral gender.
ii
MARKET AND INDUSTRY DATA
This prospectus contains estimates, projections, and other information concerning our industry and business, as well as data regarding market research, estimates, and forecasts prepared by our management. Information that is based on estimates, forecasts, projections, market research, or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. The industry in which we operate is subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section titled “Risk Factors.” Unless otherwise expressly stated, we obtained industry, business, market, and other data from reports, research surveys, studies, and similar data prepared by market research firms and other third parties, industry and general publications, government data, and similar sources. In some cases, we do not expressly refer to the sources from which this data is derived. In that regard, when we refer to one or more sources of this type of data in any paragraph, you should assume that other data of this type appearing in the same paragraph is derived from sources that we paid for, sponsored, or conducted, unless otherwise expressly stated or the context otherwise requires. While we have compiled, extracted, and reproduced industry data from these sources, we have not independently verified the data. Forecasts and other forward-looking information with respect to industry, business, market, and other data are subject to the same qualifications and additional uncertainties regarding the other forward-looking statements in this prospectus. See “Disclosure Regarding Forward-Looking Statements.”
iii
TRADEMARKS AND TRADE NAMES
We own or have rights to various trademarks, service marks and trade names that they use in connection with the operation of their respective businesses. This prospectus also contains trademarks, service marks and trade names of third parties, which are the property of their respective owners. The use or display of third parties’ trademarks, service marks, trade names or products in this prospectus is not intended to create, and does not imply, a relationship with us, or an endorsement or sponsorship by or of us. Solely for convenience, the trademarks, service marks and trade names referred to in this prospectus may appear with the ®, TM or SM symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the right of the applicable licensor to these trademarks, service marks and trade names.
iv
PRESENTATION OF FINANCIAL INFORMATION
The financial information contained in this prospectus derives from our audited consolidated financial statements in AUD$ as of June 30, 2024 and 2023 and for each of the two years then ended, and from our unaudited interim condensed consolidated financial statements in AUD$ as of December 31, 2024, and for the six months ended December 31, 2024 and 2023. These financial statements and related notes included elsewhere in this prospectus are in the form of Australian Dollar (AUD$) and are collectively referred to as our audited consolidated financial statements herein and throughout this prospectus. Our audited consolidated financial statements are prepared in accordance with International Financial Reporting Standards and International Accounting Standards as issued by the International Accounting Standards Board (IASB) and Interpretations (collectively IFRSs). Our fiscal year ends on June 30 of each year, so all references to a particular fiscal year are to the applicable year ended June 30. None of our financial statements were prepared in accordance with generally accepted accounting principles in the United States.
v
EXCHANGE RATES
Our reporting currency and functional currency is the Australian Dollar. We are not currently exposed for foreign currency risk. Foreign exchange risk arises from future commercial transactions and recognized financial assets and financial liabilities denominated in a currency that is not the entity’s functional currency. The risk is measured using sensitivity analysis and cash flow forecasting. Management understands, it will in the future, deal in foreign currencies and will have in place a risk management policy when it is required.
In this prospectus, unless otherwise stated, all references to “U.S. dollars”, “USD$,” are to the currency of the United States of America, and all references to “Australian Dollars,” “AUD$”, are to the currency of Australia. Our presentation currency of the financial statements was AUD$ and will remain AUD$. Any discrepancies in any table between totals and sums of the amounts listed are due to rounding. Certain amounts and percentages have been rounded; consequently, certain figures may add up to be more or less than the total amount and certain percentages may add up to be more or less than 100% due to rounding. In particular and without limitation, amounts expressed in millions contained in this prospectus have been rounded to a single decimal place for the convenience of readers.
All amounts set forth herein are presented in United States Dollars (USD$), unless otherwise specified, and have for presentation purposes have been converted from their AUD$ equivalent using the exchange rate of 1 AUD$ to 0.65 USD$.
vi
PROSPECTUS SUMMARY
The following summary highlights certain information about us, this offering and selected information contained elsewhere in this prospectus and is qualified in its entirety by, and should be read in conjunction with, the more detailed information and financial statements included elsewhere in this prospectus before making an investment decision. In addition to this summary, we urge you to read the entire prospectus carefully, especially the risks of investing in the Ordinary Shares, discussed under “Risk Factors,” before deciding whether to buy the Ordinary Shares.
Overview
We are a clinical and science-based company that is focused on developing and commercializing white label gel-based delivery solutions for prescription drugs, nutraceuticals, pet care and other products. A “white label” gel-based delivery solution is where we produce a product that other companies rebrand as their own product. Our principal products are edible gels, which we refer to as gels, and their application in gel-based dosage forms. Our current product suite consists of multiple products that sit within five core verticals — for pets, sports, pharmaceutical (pharma), over-the-counter (OTC) and nutraceutical — all of which leverage our patent pending multiple-ingredient dosage forms, and that we expect to have a wide range of applications and consumers. We currently focus our efforts on out-licensing our technology to companies to develop and create new products they can manufacture and sell within their established and researched markets, while we continue to manufacture our existing products under license (“white label”).
Recent Developments
On October 30, 2024, we consummated our initial public offering of 1,300,000 Ordinary Shares at a price of US$4.00 per share, generating gross proceeds to the Company of $5.2 million before deducting underwriting discounts and offering expenses. In connection with the IPO, the Company entered into an Underwriting Agreement, dated October 28, 2024 (the “Underwriting Agreement”) by and between the Company and The Benchmark Company, LLC as representative of the several underwriters. The Company agreed to an underwriting discount of 7.0% of the public offering price of the Ordinary Shares sold in the IPO.
On November 14, 2024, we entered into a license agreement for office space rental in New York for a fee of 4,468 USD per month. The license agreement has an initial term of six months and automatically renews for additional six-month terms upon the expiration of the initial term.
On December 2, 2024, we entered into an agreement with WPIC Marketing and Technologies Limited (“WPIC”) to assist with sales and distribution of our SportsGel products throughout the Asian Pacific region, commencing with China initially in March 2025. As of the date of this prospectus, we have four online stores across various platforms open in China as WPIC’s first region of focus.
On December 19, 2024, we appointed Dr. Paul Wynne as our Chief Scientific Officer.
On March 31, 2025, Simon H. Szewach resigned as our Executive Chairman. He continues to serve as our Chairman and Director.
On April 30, 2025, David A.V. Morton resigned as a Director.
On June 3, 2025, Anthony W. Panther resigned as our Chief Financial Officer and on the same day, Thuy-Linh Gigler became the Company’s Chief Financial Officer.
Lincoln Park Purchase Agreement
On March 13, 2025, we entered into the Purchase Agreement with Lincoln Park pursuant to which we have the right, but not the obligation, to sell to Lincoln Park up to $12,000,000 of Purchase Shares from time to time over the 24-month term beginning only after certain conditions set forth in the Purchase Agreement have been satisfied, including that this Registration Statement shall have been declared effective under the Securities Act, which we refer to as the Commencement Date. In accordance with the Purchase Agreement, on March 15, 2025, we issued 175,000 Ordinary Shares (the “Commitment Shares”) to Lincoln Park as consideration for its commitment to purchase our Ordinary Shares under the Purchase Agreement. On the business day immediately following the Commencement Date (the “Effective Purchase Date”), the Company has the option to sell to Lincoln Park under the Purchase Agreement an amount of Ordinary Shares equal to $50,000, at a price per share calculated on the same basis as a Regular Purchase (as defined below); provided, however, that such option will only be available on the Effective Purchase Date.
1
Although the Purchase Agreement provides that we may sell up to an aggregate of $12.0 million of our Ordinary Shares to Lincoln Park, only 4,000,000 Ordinary Shares are being registered for resale under this prospectus, which includes the 175,000 Commitment Shares that we already issued to Lincoln Park as a fee for making its irrevocable commitment to purchase our Ordinary Shares under the Purchase Agreement. Depending on the market prices of our Ordinary Shares at the time we elect to issue and sell our Ordinary Shares to Lincoln Park under the Purchase Agreement, we may need to register for resale under the Securities Act additional Ordinary Shares in order to receive aggregate gross proceeds equal to the $12.0 million total commitment available to us under the Purchase Agreement. If we elect to issue and sell to Lincoln Park under the Purchase Agreement more than the 3,825,000 Ordinary Shares being registered for resale by Lincoln Park under this prospectus, which we have the right, but not the obligation, to do, we must first register for resale under the Securities Act any such additional Ordinary Shares, which could cause additional substantial dilution to our shareholders. The number of Ordinary Shares ultimately offered for resale by Lincoln Park is dependent upon the number of Ordinary Shares we ultimately decide to sell to Lincoln Park under the Purchase Agreement.
To the extent that we are subject to the applicable rules of Nasdaq, in no event may we issue or sell to Lincoln Park under the Purchase Agreement our Ordinary Shares, including the Commitment Shares, in excess of 1,881,328 shares, which is equal to 19.99% of our Ordinary Shares outstanding immediately prior to the execution of the Purchase Agreement (the “Exchange Cap”) unless (i) we obtain shareholder approval to issue our Ordinary Shares in excess of the Exchange Cap or (ii) the average price of all Ordinary Shares issued to Lincoln Park under the Purchase Agreement equals or exceeds $1.29 per share (which represents the lower of (A) the official closing price of the Ordinary Shares on Nasdaq immediately preceding the signing of the Purchase Agreement and (B) the average official closing price of the Ordinary Shares on Nasdaq for the five consecutive trading days ending on the trading day immediately preceding the date of the Purchase Agreement), such that the transactions contemplated by the Purchase Agreement are exempt from the Exchange Cap limitation under applicable Nasdaq rules. In any event, the Purchase Agreement specifically provides that we may not issue or sell any Ordinary Shares under the Purchase Agreement if such issuance or sale would breach any applicable rules or regulations of Nasdaq or the Corporations Act 2001 (Commonwealth of Australia). The Purchase Agreement also prohibits us from directing Lincoln Park to purchase any of our Ordinary Shares if those shares, when aggregated with all other Ordinary Shares then beneficially owned by Lincoln Park (as calculated pursuant to Section 13(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and Rule 13d-3 thereunder), would result in Lincoln Park and its affiliates beneficially owning more than 4.99% of the then total Ordinary Shares (which may be increased up to 9.99% of our Ordinary Shares), which we refer to herein as the Beneficial Ownership Limitation.
Lincoln Park Registration Rights Agreement
Concurrently with entering into the Purchase Agreement, we entered into a registration rights agreement with Lincoln Park (the “Registration Rights Agreement”) pursuant to which we agreed to register the resale of our Ordinary Shares that have been and may be issued to Lincoln Park under the Purchase Agreement pursuant to this Registration Statement.
Implications of Being an “Emerging Growth Company”
As a company with less than USD$1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An “emerging growth company” may take advantage of reduced reporting requirements that are otherwise applicable to larger public companies. In particular, as an emerging growth company, we:
• may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations, or “MD&A”;
• are not required to provide a detailed narrative disclosure discussing our compensation principles, objectives and elements and analyzing how those elements fit with our principles and objectives, which is commonly referred to as “compensation discussion and analysis”;
• are not required to obtain an attestation and report from our auditors on our management’s assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002;
2
• are not required to obtain a non-binding advisory vote from our shareholders on executive compensation or golden parachute arrangements (commonly referred to as the “say-on-pay,” “say-on frequency” and “say-on-golden-parachute” votes);
• are exempt from certain executive compensation disclosure provisions requiring a pay-for-performance graph and chief executive officer pay ratio disclosure;
• are eligible to claim longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act; and
• will not be required to conduct an evaluation of our internal control over financial reporting.
We intend to take advantage of all of these reduced reporting requirements and exemptions, with the exception of the longer phase-in periods for the adoption of new or revised financial accounting standards under §107 of the JOBS Act.
Under the JOBS Act, we may take advantage of the above-described reduced reporting requirements and exemptions until we no longer meet the definition of an emerging growth company. The JOBS Act provides that we would cease to be an “emerging growth company” at the end of the fiscal year in which the fifth anniversary of our initial sale of common equity pursuant to a registration statement declared effective under the Securities Act of 1933, as amended, herein referred to as the Securities Act, occurred, if we have more than USD$1.235 billion in annual revenues, have more than USD$700 million in market value of the Ordinary Shares held by non-affiliates, or issue more than USD$1 billion in principal amount of non-convertible debt over a three-year period.
Implications of Being a Foreign Private Issuer
We are a foreign private issuer within the meaning of the rules under the Securities Exchange Act of 1934, as amended (the “Exchange Act”). As such, we are exempt from certain provisions applicable to United States domestic public companies. For example:
• we are not required to provide as many Exchange Act reports, or as frequently, as a domestic public company;
• for interim reporting, we are permitted to comply solely with our home country requirements, which are less rigorous than the rules that apply to domestic public companies;
• we are not required to provide the same level of disclosure on certain issues, such as executive compensation;
• we are exempt from provisions of Regulation FD aimed at preventing issuers from making selective disclosures of material information;
• we are not required to comply with the sections of the Exchange Act regulating the solicitation of proxies, consents, or authorizations in respect of a security registered under the Exchange Act; and
• we are not required to comply with Section 16 of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and establishing insider liability for profits realized from any “short-swing” trading transaction.
We will be required to file an annual report on Form 20-F within four months of the end of each fiscal year. In addition, we intend to publish our results on a quarterly basis as press releases, distributed pursuant to the rules and regulations of Nasdaq. Press releases relating to financial results and material events will also be furnished to the SEC on Form 6-K. However, the information we are required to file with or furnish to the SEC will be less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. As a result, you may not be afforded the same protections or information that would be made available to you were you investing in a U.S. domestic issuer.
The Nasdaq listing rules provide that a foreign private issuer may follow the practices of its home country, which for us is Australia, rather than the Nasdaq rules as to certain corporate governance requirements, including the requirement that the issuer have a majority of independent directors and the audit committee, compensation committee and nominating and corporate governance committee requirements, the requirement to disclose third party director and
3
nominee compensation and the requirement to distribute annual and interim reports. A foreign private issuer that follows a home country practice in lieu of one or more of the listing rules shall disclose in its annual reports filed with the SEC each requirement that it does not follow and describe the home country practice followed by the issuer in lieu of such requirements. Although we do not currently intend to take advantage of these exceptions to Nasdaq corporate governance rules, we may in the future take advantage of one or more of these exemptions.
Corporate Information
Our registered office is located at Vistra Australia, Level 4, 100 Albert Road, South Melbourne VIC, 3025, Australia. Our principal place of business is located at Monash Innovation Labs, G. 60, 22 Alliance Lane, Clayton 3800, Victoria, Australia and our telephone number is +61 3 9087 3990. Our website address is http://www.gelteq.com. The information contained therein, or that can be accessed therefrom, is not and shall not be deemed to be incorporated into this prospectus or the registration statement of which it forms a part.
Corporate History and Structure
We were incorporated under the laws of the State of Victoria, Australia on October 15th, 2018. Our technology was assigned to us by our founders and a predecessor entity, who created it prior to the incorporation of our company. The intellectual property was then assigned to Gelteq at Gelteq’s inception to continue to build on this work.
We currently have three direct, wholly-owned subsidiaries as part of our organizational structure: Nutrigel Pty Ltd and Unit Trust (“NPL”), Sport Supplements Pty Ltd and Unit Trust (“SSPL”) and Gelteq US Inc, a Delaware corporation.
The chart below summarizes our corporate structure, including our direct, wholly-owned subsidiaries, as of the date of this prospectus:
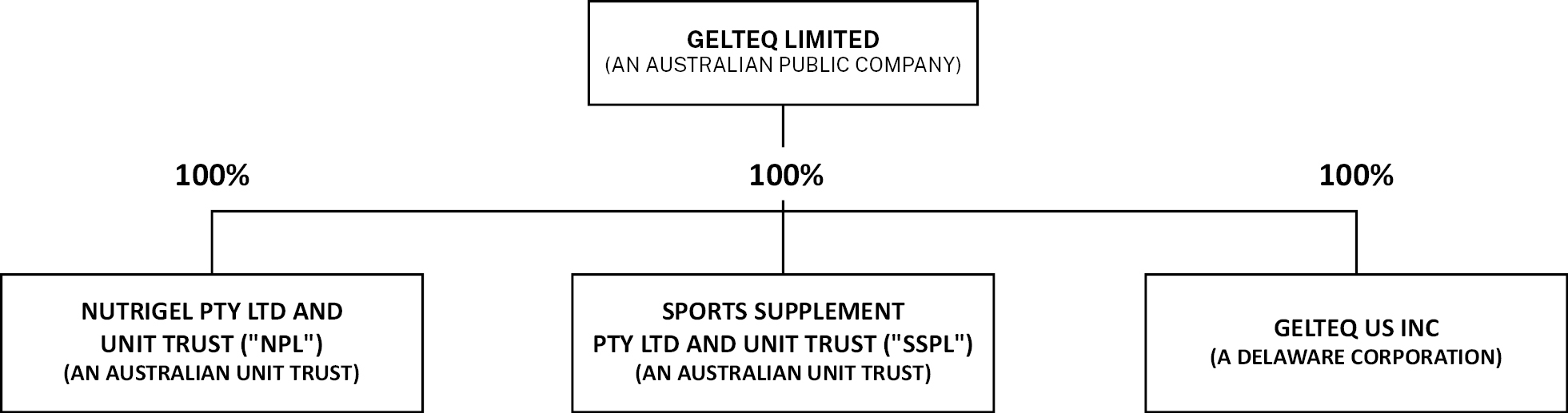
4
THE OFFERING
The summary below describes the principal terms of the offering of our Ordinary Shares. This summary is not complete and does not contain all the information you should consider before investing in our Ordinary Shares. You should carefully read this entire prospectus before investing in our Ordinary Shares including the sections “The Committed Equity Financing,” and “Risk Factors.”
|
Ordinary Shares Offered by the Selling Shareholder |
• up to 3,825,000 Ordinary Shares which we may sell to Lincoln Park from time to time over the next 24 months beginning on the Commencement Date, at our sole discretion, in accordance with the Purchase Agreement; and • 175,000 Ordinary Shares previously issued to Lincoln Park as consideration for its commitment to purchase our Ordinary Shares under the Purchase Agreement. We did not receive any cash proceeds from the issuance of these Commitment Shares. |
|
|
Ordinary Shares outstanding immediately after this offering(1) |
|
|
|
Nasdaq Listing |
Our Ordinary Shares currently traded on the Nasdaq Capital Market under the symbol “GELS.” |
|
|
Use of Proceeds |
We will receive no proceeds from the sale of the Ordinary Shares by Lincoln Park in this offering. However, we may receive up to $12,000,000 in aggregate gross proceeds under the Purchase Agreement from any sales of the Ordinary Shares from time to time after the date that the registration statement of which this prospectus is a part is declared effective and after satisfaction of other conditions in the Purchase Agreement. Depending on the market prices of our Ordinary Shares at the time we elect to issue and sell our Ordinary Shares to Lincoln Park under the Purchase Agreement, we may need to register for resale under the Securities Act additional Ordinary Shares in order to receive aggregate gross proceeds equal to the $12.0 million total commitment available to us under the Purchase Agreement. We currently intend to use any proceeds that we receive from the sale of Ordinary Shares to Lincoln Park under the Purchase Agreement for research and development, marketing activities and general working capital. |
|
|
Risk Factors |
See “Risk Factors” for a discussion of risks you should carefully consider before investing in the Ordinary Shares. |
____________
(1) The number of Ordinary Shares outstanding after this offering is based on the number of shares outstanding as of June 27, 2025, and excludes 91,000 of our Ordinary Shares issuable upon the exercise of underwriter’s warrants, issued to The Benchmark Company LLC as representative of the underwriters for our IPO, outstanding as of December 31, 2024 at an exercise price of $5.00 per Ordinary Share.
5
SUMMARY FINANCIAL DATA
The following tables set forth selected historical financial data for our business. The selected historical financial data (in AUD$) for our business is taken from our audited consolidated financial statements which have been prepared in accordance with International Financial Reporting Standards and International Accounting Standards as issued by the International Accounting Standards Board (IASB) and Interpretations (collectively IFRSs). Our historical results are not necessarily indicative of the results that may be expected in the future. You should read this data together with our audited consolidated financial statements and related notes appearing elsewhere in this prospectus as well as “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” appearing elsewhere in the prospectus.
We have derived the summary statements of loss and comprehensive loss data for the years ended June 30, 2024 and June 30, 2023 and for the six months ended December 31, 2024 and December 31, 2023, and the summary statement of financial position data as at June 30, 2024 and June 30, 2023, from our audited consolidated financial statements as at June 30, 2024 and as at June 30, 2023, and for the two years then ended, and unaudited condensed consolidated financial statements as at December 31, 2024, and for the six months ended December 31, 2024 and 2023, included elsewhere in this prospectus.
Summary Statement of Consolidated Profit or Loss:
|
For the year ended |
For the six months ended |
|||||||||||
|
2024 |
2023 |
2024 |
2023 |
|||||||||
|
Revenue |
|
|
|
|
||||||||
|
Revenue from contracts with customers |
— |
|
79,843 |
|
— |
|
— |
|
||||
|
Gains from loan modifications |
— |
|
222,681 |
|
— |
|
— |
|
||||
|
Other income |
146,884 |
|
317,888 |
|
311,412 |
|
76,879 |
|
||||
|
Expenses |
|
|
|
|
||||||||
|
Raw materials and consumable expenses |
— |
|
(48,925 |
) |
— |
|
— |
|
||||
|
Advertising & marketing expense |
(18,200 |
) |
(166,929 |
) |
(118,211 |
) |
(18,200 |
) |
||||
|
Consulting fees |
(750 |
) |
(80,407 |
) |
(409,134 |
) |
(750 |
) |
||||
|
Depreciation and amortization expenses |
(1,211,896 |
) |
(1,226,491 |
) |
(606,497 |
) |
(609,274 |
) |
||||
|
Employee benefit expense |
(875,579 |
) |
(752,584 |
) |
(248,655 |
) |
(519,687 |
) |
||||
|
Finance costs |
(600,220 |
) |
(404,069 |
) |
(620,785 |
) |
(286,791 |
) |
||||
|
IPO related expenses |
(166,084 |
) |
(278,319 |
) |
(637,594 |
) |
(102,941 |
) |
||||
|
Legal Fees |
— |
|
(5,270 |
) |
— |
|
— |
|
||||
|
Research expense |
(276,057 |
) |
(665,035 |
) |
(314,472 |
) |
(100,934 |
) |
||||
|
Share based expense |
— |
|
— |
|
— |
|
— |
|
||||
|
Other expenses |
(145,851 |
) |
(69,681 |
) |
(203,680 |
) |
(25,527 |
) |
||||
|
Corporate expenses |
(222,641 |
) |
(428,922 |
) |
(456,743 |
) |
(98,419 |
) |
||||
|
Inventory Write Off |
(175,081 |
) |
— |
|
— |
|
— |
|
||||
|
Intellectual Property Services |
— |
|
— |
|
— |
|
— |
|
||||
|
(Loss) before income tax |
(3,546,195 |
) |
(3,506,220 |
) |
(3,304,359 |
) |
(1,685,644 |
) |
||||
|
Tax income/(expense) |
|
|
— |
|
— |
|
||||||
|
(Loss) after income tax |
(3,546,195 |
) |
(3,506,220 |
) |
(3,304,359 |
) |
(1,685,644 |
) |
||||
|
Weighted average number of Ordinary Shares – basic and diluted |
7,940,026 |
|
7,940,026 |
|
8,620,569 |
|
8,118,075 |
|
||||
|
Loss per share attributable to owners of the company – basic and diluted |
(0.44 |
) |
(0.44 |
) |
(0.38 |
) |
(0.21 |
) |
||||
6
Summary Statement of Consolidated Comprehensive Income:
|
For the year ended |
For the six months ended |
|||||||||||
|
2024 |
2023 |
2024 |
2023 |
|||||||||
|
(Loss) |
(3,546,195 |
) |
(3,506,220 |
) |
(3,304,359 |
) |
(1,685,644 |
) |
||||
|
Other comprehensive income |
|
|
|
|
||||||||
|
Total other comprehensive income |
— |
|
— |
|
— |
|
— |
|
||||
|
Total comprehensive (expense) |
(3,546,195 |
) |
(3,506,220 |
) |
(3,304,359 |
) |
(1,685,644 |
) |
||||
|
Total comprehensive (expense) attributable to members of the company |
(3,546,195 |
) |
(3,506,220 |
) |
(3,304,359 |
) |
(1,685,644 |
) |
||||
Summary Statement of Financial Position Data:
|
As at |
As at |
As at |
As at |
|||||||||
|
ASSETS |
|
|
|
|
||||||||
|
Current Assets |
|
|
|
|
||||||||
|
Cash and cash equivalents |
24,522 |
|
399,224 |
|
3,046,602 |
|
24,522 |
|
||||
|
Trade and other receivables |
183,004 |
|
345,291 |
|
305,007 |
|
183,004 |
|
||||
|
Inventories |
— |
|
95,201 |
|
— |
|
— |
|
||||
|
Prepayments and other assets |
95,700 |
|
151,258 |
|
1,587,521 |
|
95,700 |
|
||||
|
Total Current Assets |
303,227 |
|
990,974 |
|
4,939,130 |
|
303,227 |
|
||||
|
|
|
|
|
|||||||||
|
Non-Current Assets |
|
|
|
|
||||||||
|
Plant and equipment |
16,642 |
|
— |
|
18,056 |
|
16,642 |
|
||||
|
Right-of-use assets |
— |
|
10,001 |
|
— |
|
— |
|
||||
|
Intangible Assets |
20,437,958 |
|
21,493,661 |
|
20,158,270 |
|
20,437,958 |
|
||||
|
Total Non-Current Assets |
20,454,600 |
|
21,503,662 |
|
20,176,326 |
|
20,454,600 |
|
||||
|
Total Assets |
20,757,827 |
|
22,494,636 |
|
25,115,456 |
|
20,757,827 |
|
||||
|
|
|
|
|
|||||||||
|
LIABILITIES |
|
|
|
|
||||||||
|
Current Liabilities |
|
|
|
|
||||||||
|
Trade and other payables |
1,558,186 |
|
1,184,404 |
|
892,791 |
|
1,558,186 |
|
||||
|
Deferred Revenue |
125,359 |
|
85,359 |
|
118,704 |
|
125,359 |
|
||||
|
Borrowings, net |
2,084,152 |
|
5,086 |
|
3,882,778 |
|
2,084,152 |
|
||||
|
Derivative liability |
— |
|
— |
|
1,279,184 |
|
— |
|
||||
|
Lease liabilities |
— |
|
11,896 |
|
— |
|
— |
|
||||
|
Employee benefit provisions |
98,368 |
|
77,780 |
|
105,198 |
|
98,368 |
|
||||
|
Total Current Liabilities |
3,866,065 |
|
1,364,525 |
|
6,278,655 |
|
3,866,065 |
|
||||
|
|
|
|
|
|||||||||
|
Non-Current Liabilities |
|
|
|
|
||||||||
|
Borrowings |
1,759,447 |
|
2,471,619 |
|
13,550 |
|
1,759,447 |
|
||||
|
Lease liabilities |
— |
|
— |
|
|
|
— |
|
||||
|
Employee benefit provisions |
20,018 |
|
— |
|
29,488 |
|
20,018 |
|
||||
|
Total Non-Current Liabilities |
1,779,465 |
|
2,471,619 |
|
43,038 |
|
1,779,465 |
|
||||
|
Total Liabilities |
5,645,530 |
|
3,836,144 |
|
6,321,693 |
|
5,645,530 |
|
||||
|
Net Assets |
15,112,297 |
|
18,658,492 |
|
18,793,763 |
|
15,112,297 |
|
||||
|
|
|
|
|
|||||||||
|
EQUITY |
|
|
|
|
||||||||
|
Issued capital |
26,608,227 |
|
26,608,227 |
|
33,594,052 |
|
26,608,227 |
|
||||
|
Reserves |
|
|
— |
|
|
|
|
|
||||
|
Accumulated losses |
(11,495,930 |
) |
(7,949,735 |
) |
(14,800,289 |
) |
(11,495,930 |
) |
||||
|
Total Equity (Deficit) |
15,112,297 |
|
18,658,492 |
|
18,793,763 |
|
15,112,297 |
|
||||
7
RISK FACTORS
An investment in the Ordinary Shares involves a high degree of risk. Before deciding whether to invest in the Ordinary Shares, you should consider carefully the risks described below, and in “Item 3. Key Information — D. Risk factors” in our Annual Report on Form 20-F for the year ended June 30, 2024, incorporated by reference herein, together with all of the other information set forth in this prospectus, including the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operation” and our consolidated financial statements and related notes. If any of these risks actually occurs, our business, financial condition, results of operations or cash flow could be materially and adversely affected, which could cause the trading price of the Ordinary Shares to decline, resulting in a loss of all or part of your investment. Further, if we fail to meet the expectations of the public market in any given period, the market price of the Ordinary Shares could decline. Our business involves significant risks and uncertainties, some of which are outside of our control. If any of these risks actually occurs, our business and financial condition could suffer and the price of the Ordinary Shares could decline. The risks described below are not the only ones that we face. Additional risks not presently known to us or that we currently deem immaterial may also affect our business. You should only consider investing in the Ordinary Shares if you can bear the risk of loss of your entire investment.
Risks Related to Our Business and Industry
We have a history of operating losses and may not achieve or sustain profitability in the future
We have incurred operating losses since our inception and expect to continue to incur operating losses for the foreseeable future. We have recently commenced marketing our products and cannot be sure we will be able to continue to increase our sales to achieve profitability. Our ability to achieve profitability depends on a number of factors, including our ability to successfully market our existing products, directly or through partners, continue to develop new products, obtain regulatory approval for our products, as necessary and consummate partnership and licensing agreements.
We expect to continue to incur losses for the foreseeable future, and these losses will likely increase as we:
• develop new products;
• complete testing of products that we have created;
• clinical trials can offer take longer than expected and be more costly than originally budgeted for;
• negotiate partnerships and licensing arrangements with respect our products;
• implement internal systems and infrastructures;
• hire management and other personnel; and
• ramp up our sales and marketing infrastructure and operations to drive sales of our products.
If we are unsuccessful in developing products or if our products do not achieve market acceptance, we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations and cash flows. Moreover, our prospects must be considered in light of the risks and uncertainties encountered by an early-stage company and in highly regulated and competitive markets, such as the drug delivery market, where regulatory approval and market acceptance of our products are uncertain. There can be no assurance that our efforts will ultimately be successful or result in revenues or profits.
We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed could force us to delay, limit, reduce or terminate our product development or commercialization efforts.
We incurred total losses in the past of approximately AUD$3,546,195 and AUD$3,506,220 in the fiscal years ended June 30, 2024 and 2023 respectively and approximately AUD$ 3,304,359 and AUD$ 1,685,644 for the six months ended December 31, 2024 and 2023 respectively. Our ability to achieve and sustain profitability in the future depends in part on the rate of growth of, and changes in technology trends in, our market; the global economy; our ability to develop new products and technologies in a timely manner; the competitive position of our products; our ability to
8
manage our operating expenses; and other factors and risks, some of which are described in this prospectus. We may also seek to increase our operating expenses and make additional expenditures in anticipation of generating higher revenues, which we may not realize, if at all, until sometime in the future. As such, there can be no assurance that we will be able to achieve or sustain profitable operations in the future.
We have expended and believe that, subject to receiving adequate financing and/or entering into a collaboration agreement, we will continue to expend significant operating and capital expenditures for the foreseeable future developing, establishing licensing and partnership arrangement and marketing our products. These expenditures will include, but are not limited to, costs associated with research and development, manufacturing, conducting studies of new products and product applications, contracting with research organizations, obtaining and retaining development, sales and marketing partnerships and hiring additional management and other personnel. We cannot reasonably estimate the actual amounts necessary to successfully complete the research, development and commercialization of our products and any other future product. In addition, other unanticipated costs may arise. As a result of these and other factors currently unknown to us, we will require additional funds, through public or private equity or debt financings or other sources, such as strategic partnerships and alliances and licensing arrangements. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. A failure to fund these activities may harm our growth strategy, competitive position, quality compliance and financial condition.
Our future capital requirements depend on many factors, including:
• the scope, progress, results and costs of researching and developing our products;
• the cost of manufacturing our products;
• our ability to establish and maintain strategic partnerships, licensing, supply or other arrangements and the financial terms of such agreements;
• the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, including litigation costs and the outcome of such litigation;
• the expenses needed to attract and retain skilled personnel; and
• any product liability or other lawsuits related to existing and/or any future products.
Additional funds may not be available when we need them, on terms that are acceptable to us, or at all. If adequate funds are not available to us on a timely basis, we may be required to delay, limit, reduce or terminate research and development activities for our products or delay, limit, reduce or terminate our establishment of sales and marketing capabilities or other activities that may be necessary to commercialize our products.
Our operating results may fluctuate, as we have created a new class of products for which demand is unknown, which makes our results difficult to predict and could cause our results to fall short of our expectations.
Our principal products are edible gels, which we refer to as gels, and their application in gel-based dosage forms. While other companies manufacture and sell edible gels, we believe we are the first company to market edible gels in many of the verticals industries we are targeting. Going forward, our operating results may fluctuate as a result of a number of factors, including, without limitation, the costs associated with raw materials, manufacturing costs and expenses and the costs incurred in our marketing and distribution and sales network, many of which are outside of our control. As a result, comparing our operating results on a period-to-period basis may not be meaningful, and you should not rely on our past results as an indication of our future performance. Our interim, year-to-date, and annual expenses as a percentage of our revenues may differ significantly over time. Our operating results in future quarters may fall below expectations.
Because our business is changing and evolving, our historical and current operating results may not be useful to you in predicting our future operating results.
9
Fluctuations in the prices of raw materials can increase the cost of our products, impact our ability to meet production commitments, and may adversely affect our results of operations.
The cost of raw materials is a key element in the cost of our gels. Our inability to offset material price inflation through increased prices to customers and suppliers, or through productivity actions could adversely affect our results of operations. Many major components, product equipment items, and raw materials are procured or subcontracted, which may negatively affect the availability and price of essential aspects of our products. Our inability to fill our supply needs would jeopardize our ability to fulfill obligations under our contracts, which could, in turn, result in reduced sales and profits, contract penalties or terminations, and damage to our customer and distributor relationships. The cost of raw materials that are applied to manufacture our products has been impacted and is expected to continue to be impacted by the risks we may face arising from the Russian invasion of Ukraine.
Our customers have a history of delaying orders which may adversely affect our revenues and income.
We have received several orders from customers who subsequently advised us of cash flow difficulties and their inability to pay for such orders in a timely manner. This has limited our ability to generate the expected revenue off these orders in a timely manner. However, as of the date of this prospectus. such customers that experienced cash flow difficulties had not cancelled their orders and we have manufactured such orders and shipped them in the fiscal year ending June 30, 2025. We have put in place more rigorous qualification procedures to ensure customers have the financial ability to pay for its orders. However, we cannot guarantee that our customers will present their accurate business status to us and pay for their orders in a timely order, which may adversely affect our revenues and income.
There is substantial doubt about our ability to continue as a going concern.
Our audited financial statements for the years ended June 30, 2024 and 2023, and our unaudited financial statements for the six months ended December 31, 2024, were prepared assuming that we will continue as a going concern. In addition, as discussed in Note 4 of the financial statements for the years ended June 30, 2024 and 2023, and the six months ended December 31, 2024, the Company was in a current liability position as of December 31, 2024, June 30, 2024 and 2023, and has suffered recurring losses from operations. These conditions raise substantial doubt on our ability to continue as a going concern. The report of our independent registered public accounting firms on the financial statements for the year ended June 30, 2024 and 2023 both included an explanatory paragraph on the doubt of our ability to continue as a going concern.
As discussed in Note 4 of the financial statements for the years ended June 30, 2024 and 2023, and for the six months ended December 31, 2024, our ability to continue as a going concern will be dependent upon our management’s plans and execution, which includes raising additional capital, either through the proposed offering or raising funds through private markets or the issue of additional Convertible Notes, obtaining regulatory approvals for our products and generating revenues from these products and reducing expenditure accordingly if required, in order to be able to pay its debts as and when they fall due. If we fail to obtain regulatory approval for our products or fail to raise additional capital in debt or equity financing on terms favorable to us, then we may be unable to achieve our objectives. Further, subsequent to the Company’s board of directors signing of the June 30, 2023 and 2022 financial statements in December 2023, the shareholder loans, issued on January 20, 2022 collectively have maturity dates of December 31, 2025 and such loans will become due within 12 months from December 31, 2024. As such, our ability to continue as a going concern is also dependent on our management’s ability to either extend the maturity date of such shareholder loans or have the shareholders elect to convert such loans into equity.
Our business and our ability to raise capital may be materially adversely affected by global geopolitical conditions resulting from the ongoing Russia-Ukraine conflict, the Israel-Hamas conflict and recent actions undertaken by the United States, such as the imposition of tariffs and the response of China and other nations thereto.
Global markets are experiencing volatility and disruption as a result of the geopolitical instability resulting from the ongoing Russia-Ukraine conflict, the Israel-Hamas conflict and recent actions undertaken by the United States, such as the imposition of tariffs and the response of China and other nations thereto. The invasion of Ukraine by Russia, the Israel-Hamas conflict, recent actions undertaken and threatened by the United States and the resulting measures that have been taken, and could be taken in the future, by China, NATO, the United States, the United Kingdom, the European Union, Israel and other countries have created global security concerns that could have a lasting impact on regional and global economies and financial markets. Although the length and impact of the ongoing conflicts
10
are highly unpredictable, they could lead to market disruptions, including significant volatility in commodity prices, credit and capital markets, as well as supply chain interruptions. Additionally, any resulting sanctions could adversely affect the global economy and financial markets and lead to instability and lack of liquidity in capital markets. The uncertainty regarding the imposition of tariffs could cause large variations in shipping costs and timelines, higher import costs and costs of goods as well as increased uncertainty around customs clearance timelines and costs.
Any of the abovementioned factors, or any other negative impact on the global economy, capital markets or other geopolitical conditions resulting from such actions, could adversely affect our business or disrupt the capital markets, impacting our ability to raise capital. The extent and duration of the ongoing conflicts, resulting sanctions and any related market disruptions are impossible to predict, but could be substantial, particularly if current or new sanctions continue for an extended period of time or if geopolitical tensions result in expanded military operations on a global scale. Additionally, the uncertainty on tariffs increases the risk that we may not be able to hedge our pricing nor derive the margins we plan to achieve. Any such disruptions may also have the effect of heightening many of the other risks described in this section. If these disruptions or other matters of global concern continue for an extensive period of time, our business may be materially adversely affected.
If the market for our gels does not develop or become sustainable, expands more slowly than we expect, or becomes saturated, our revenues may fail to materialize, and our financial condition and results of operations could be materially and adversely affected.
The market for our products is new and rapidly evolving, and we may face an unexpected number of competitors. We believe that our innovative gel products are addressing a market that did not exist previously and there is no assurance that the gel industry will develop as envisioned by us, or that, if it does develop, we will succeed in executing our business plan, or acquiring any meaningful market share. Our success is highly dependent on the market’s acceptance of our technology and our products, and on our leadership of any market that materializes. If the market for our products does not materialize, become sustainable, or becomes saturated with competing products or services, our revenues may not materialize, or may be lower than projections, and our financial condition and results of operations could be materially and adversely affected. Should lower than expected sales occur, we intend to adjust our expenses to align with the revenue generated to ensure we remain financially solvent and as a going concern.
Our success depends on our ability to obtain market acceptance for our products and services.
Our future success and the planned growth and expansion of our business depend on us achieving broad acceptance of our products and growing our customer base. This depends, in part, on our technology, our ability to respond to consumer preferences, our marketing plans, our ability to locate and enter into agreements with partners and adoption of our products. If we are unable to obtain customer acceptance, to effectively market our products directly or through partners, our business and results of operations will be materially impaired.
The loss of the services of our key personnel would negatively affect our business.
Our future success depends to a large extent on the continued services of our senior management and key personnel, including, in particular, our Chief Executive Officer, Nathan J. Givoni. Any loss of the services of our key personnel, and especially that of Mr. Givoni, would adversely affect our business. We have attempted to mitigate this situation by ensuring that Mr. Givoni provides us long notice periods and has extra share compensation via the employee stock option plan to encourage his long term tenure and performance with the Company. The employment agreements entered into with Mr. Givoni stipulates that he must give six months written notice of his intent to resign, allowing the Company time to find a suitable replacement.
Although our Ordinary Shares began trading on The Nasdaq Stock Market LLC on October 28, 2024, we do not know whether an active, liquid trading market for our Ordinary Shares will be sustained or what the trading price of our Ordinary Shares will be in the future. Our Ordinary Shares may trade at a price below the price you paid and may be difficult for you to sell the Ordinary Shares you purchase.
Although our Ordinary Shares are listed on Nasdaq and began trading on October 28, 2024, an active trading market for our Ordinary Shares may not be sustained. It may be difficult for you to sell your Ordinary Shares without depressing the market price for the Ordinary Shares or at all. Consequently, you may not be able to sell your Ordinary Shares at
11
or above the price you paid, or at all. Further, an inactive market may also impair our ability to raise capital by selling Ordinary Shares and it also may impair our ability to enter into strategic partnerships or acquire companies, products, or services by using our equity securities as consideration.
We cannot predict the extent to which investor interest in us will sustain an active trading market or how active and liquid that market may become in the future. If an active and liquid trading market does not continue, you may have difficulty selling your Ordinary Shares at an attractive price or at all. The market price of our Ordinary Shares may decline below the price you paid and you may not be able to sell your Ordinary Shares at or above the price you paid, or at all.
The market price of our Ordinary Shares has been and may continue to be highly volatile, and you could lose all or part of your investment.
In addition, the market price of our Ordinary Shares could fluctuate significantly as a result of a number of factors, including:
• fluctuations in our financial performance;
• economic and stock market conditions generally and specifically as they may impact us, participants in our industry or comparable companies;
• changes in financial estimates and recommendations by securities analysts following our Ordinary Shares or comparable companies;
• earnings and other announcements by, and changes in market evaluations of, us, participants in our industry or comparable companies;
• our ability to meet or exceed any future earnings guidance we may issue;
• changes in business or regulatory conditions affecting us, participants in our industry or comparable companies;
• changes in accounting standards, policies, guidance, interpretations or principles;
• announcements or implementation by our competitors or us of acquisitions, technological innovations, or other strategic actions by our competitors; or
• trading volume of our Ordinary Shares or sales of shares by our management team, directors or principal shareholders.
These and other factors could limit or prevent investors from readily selling their Ordinary Shares or otherwise negatively affect the liquidity of our Ordinary Shares, and you could lose all or part of your investment.
The market price of our Ordinary Shares could be adversely affected by future sales, including sales by the selling shareholder named herein and distributions of our Ordinary Shares or the perception that such sales, including sales by the selling shareholder named herein.
Sales, by the selling shareholder named herein, distributions or issuances of a substantial number of our Ordinary Shares following this offering or the perception that such sales or distributions might occur, could cause a decline in the market price of our Ordinary Shares or could impair our ability to obtain capital through a subsequent offering of our equity securities or securities convertible into equity securities.
We may issue additional securities in the future, including Ordinary Shares, and options, rights, warrants and other convertible securities for any purpose and for such consideration and on such terms and conditions we may determine appropriate or necessary, including in connection with equity awards, financings or other strategic transactions. Subject to the requirements of the Corporations Act, our board of directors will be able to determine the class, designations, preferences, rights and powers of any additional shares, including any rights to share in our profits, losses and dividends or other distributions, any rights to receive assets upon our dissolution or liquidation and any redemption, conversion and exchange rights.
12
Future sales of a substantial number of our Ordinary Shares by our existing shareholders in addition to the shares offered by this prospectus could cause our stock price to decline.
As of June 27, 2025, there were 10,028,025 of our Ordinary Shares outstanding. All of the Ordinary Shares sold in the IPO became eligible for sale immediately upon issuance in the IPO. Additional shares will be eligible for sale in the public market upon expiration of the remaining unexpired lock-up agreements entered into in connection with the IPO. Subject to any applicable lock-up agreements, pursuant to Rule 144 under the Securities Act as in effect on the date hereof, or Rule 144, a person who holds restricted Ordinary Shares (assuming there are any restricted shares) and is not one of our affiliates at any time during the three months preceding a sale, and who has beneficially owned these restricted shares for at least six months, would be entitled to sell an unlimited number of Ordinary Shares, provided current public information about us is available. In addition, under Rule 144, a person who holds restricted shares in us and is not one of our affiliates at any time during the three months preceding a sale, and who has beneficially owned these restricted shares for at least one year, would be entitled to sell an unlimited number of shares without regard to whether current public information about us is available. It is conceivable that following the holding period, many shareholders may wish to sell some or all of their shares. If our shareholders sell substantial amounts of our Ordinary Shares in the public market at the same time, the market price of our Ordinary Shares could decrease significantly due to an imbalance in the supply and demand of our Ordinary Shares. Even if they do not actually sell the Ordinary Shares, the perception in the public market that our shareholders might sell significant Ordinary Shares could also depress the market price of our Ordinary Shares.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they publish negative reports regarding our business or our securities, our share price and trading volume could decline.
The trading market for the Ordinary Shares will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding the Ordinary Shares, or provide more favorable relative recommendations about our competitors, the price of our Ordinary Shares would likely decline. If any analyst who may cover us were to cease coverage of our Company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our Ordinary Shares or trading volume to decline.
Management has broad discretion as to the use of proceeds we may receive from the sale of Purchase Shares to Lincoln Park.
Our management has broad discretion in the allocation of proceeds we may receive from the sale of Purchase Shares to Lincoln Park and could use such proceeds for purposes other than those contemplated at the date of this prospectus. Our shareholders may not agree with the manner in which our management chooses to allocate and spend such proceeds.
We may need additional capital beyond the capital raised from the sale of Purchase Shares to Lincoln Park, and the sale of additional Ordinary Shares or equity or debt securities could result in additional dilution to our shareholders.
Although the net proceeds from our IPO and the sale of Purchase Shares to Lincoln Park are anticipated to be sufficient for research and development, marketing activities and general working capital purposes, further funding may be required. We may need to raise additional capital beyond the capital raised in the IPO and from the sale of Purchase Shares to Lincoln Park in order to support additional growth opportunities or to provide additional cash flow should our sales not be achieved as forecasted. Such additional capital may be raised through a combination of private and public equity offerings, debt financings and collaborations, and strategic and licensing arrangements. To the extent that we raise additional capital through the issuance of equity or convertible debt securities, your ownership interest will be diluted, and the terms may include liquidation or other preferences that adversely affect your rights as a holder of our Ordinary Shares. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take certain actions, such as incurring debt, without prior approval, making capital expenditures or declaring dividends. If we raise additional funds through strategic partnerships and alliances and licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies or product or grant licenses on terms that
13
are not favorable to us. If we are unable to raise additional funds through equity or debt financing when needed, we may be required to delay, limit, reduce or terminate our product development, sales launch or marketing efforts or grant rights to develop and market product that we would otherwise prefer to develop and market ourselves.
The terms of the Purchase Agreement limit the amount of Ordinary Shares we may issue to Lincoln Park, which may limit our ability to utilize the arrangement to enhance our cash resources.
The Purchase Agreement includes restrictions on our ability to sell Ordinary Shares to Lincoln Park, including, subject to specified limitations, if a sale would cause Lincoln Park and its affiliates to exceed the Beneficial Ownership Limitation.
Accordingly, we cannot guarantee that we will be able to sell all 3,825,000 Purchase Shares in this offering. If we cannot sell the full amount of Ordinary Shares that Lincoln Park has committed to purchase because of these limitations, we may be required to utilize more costly and time-consuming means of accessing the capital markets, which could materially adversely affect our liquidity and cash position. If we choose to sell more Ordinary Shares than are offered under this prospectus, we must first register for resale under the Securities Act such additional Ordinary Shares.
If we fail to develop or maintain an effective system of disclosure controls and internal control over financial reporting in compliance with the requirements that will be applicable to us as a public company in the United States, our ability to produce timely and accurate consolidated financial statements or comply with applicable regulations could be impaired and our listing on Nasdaq Capital Market could be terminated.
As a public company in the United States, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, and the rules and regulations of the applicable listing standards of Nasdaq Capital Market. We expect that the requirements of these rules and regulations will continue to increase our legal, accounting, and financial compliance costs, make some activities more difficult, time-consuming, and costly and place significant strain on our personnel, systems, and resources. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by using the reports that we will file with the SEC is recorded, processed, summarized, and reported within the time periods specified in SEC rules and forms and that information required to be disclosed in reports under the Exchange Act is accumulated and communicated to our principal executive and financial officers.
In connection with the finalization of our consolidated audited financial statements for the year ended June 30, 2021, the Company concluded, and our independent auditors UHY Haines Norton Sydney concurred, that a material weakness existed in our internal control over financial reporting relating to several factors, which involved a lack of non-executive directors and the heavy reliance on an external accountant.
For the year ended June 30, 2021, we had no independent board members, nor an audit committee, to evaluate related party transactions against a formal benchmarking standard to determine whether such transactions were conducted at arm’s length. As a result, there were delays with transactions being reported to the Australian Securities & Investments Commission (“ASIC”). The Company had also limited internal control processes and operational checklists on business operation changes and financial reporting.
Through the financial year ended June 30, 2022, we implemented several measures to remediate these deficiencies, including appointing an independent director in August 2021 and two further independent directors to our board of directors in April 2022, hiring a Chief Financial Officer in June 2022 to implement changes to our finance function to ensure compliance and establishment of proper operational controls, reporting procedures and policies to safeguard assets and minimize financial and commercial risks, and formalizing processes for related party transactions. However, as many of these remedies occurred towards the end of the June 30, 2022 reporting period, the material weaknesses existed as part of our financial year audit report as at June 30, 2022.
In connection with finalizing our consolidated audited financial statements for the year ended June 30, 2023, the Company concluded, and our independent auditors UHY Haines Norton Sydney concurred, that the Company’s measures implemented in late 2022 to work to mitigate risks in regards to segregation of duties, having clear job roles and responsibilities, and overarching review of the financial information, had not performed as expected and therefore there are still material weaknesses through the financial year ended June 30, 2023. The Chief Financial Officer, hired in June 2022, ended his employment in February 2023 by mutual agreement, and a New Chief Financial
14
Officer was hired in February 2023. Through the financial year ended June 30, 2023, there continued to be a lack of audit committee, and whilst the Company implemented review and oversight of financial information including the financial statements and segregation of duties, these were not at a level that was effective to overcome the material weaknesses. The Company would need to further effectively segregate duties and have greater review and oversight of financial information and financial statements. The Company implemented sufficient remedial measures for the financial year ended June 30, 2024 that a material weakness did not exist and it expects to further improve on such measure for the financial year ending June 30, 2025 but there is no assurance that such remedial measures would allow us to maintain sufficient and effective disclosure controls, and procedures and internal control over financial reporting.
Any failure to develop or maintain effective controls or any difficulties encountered in their implementation or improvement could harm our results of operations or cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. Any failure to implement and maintain effective internal control over financial reporting also could adversely affect the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that may be required to include in our periodic reports that will be filed with the SEC. Ineffective disclosure controls and procedures and internal control over financial reporting could also cause investors to lose confidence in our reported financial and other information, which would likely have a negative effect on the trading price of our Ordinary Shares. In addition, if we are unable to continue to meet these requirements, we may not be able to remain listed on Nasdaq Capital Market. We are required to comply with the SEC rules that implement Section 404 of the Sarbanes-Oxley Act and are required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. Our independent registered public accounting firm is not required to formally attest to the effectiveness of our internal control over financial reporting until after we are no longer an “emerging growth company” as defined in the JOBS Act. At such time, our independent registered public accounting firm may issue a report that is adverse in the event it is not satisfied with the level at which our internal control over financial reporting is documented, designed, or operating. Any failure to maintain effective disclosure controls and internal control over financial reporting could have an adverse effect on our business and results of operations and could cause a decline in the price of our Ordinary Shares.
15
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements that reflect our current expectations and views of future events, all of which are subject to risks and uncertainties. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements by the fact that they do not relate strictly to historical or current facts. You can find many (but not all) of these statements by the use of words such as “approximates,” “believes,” “hopes,” “expects,” “anticipates,” “estimates,” “projects,” “intends,” “plans,” “will,” “would,” “should,” “could,” “may” or other similar expressions in this prospectus. These statements are likely to address our growth strategy, financial results and product research and development programs. You must carefully consider any such statements and should understand that many factors could cause actual results to differ from our forward-looking statements. These factors may include inaccurate assumptions and a broad variety of other risks and uncertainties, including some that are known and some that are not. No forward-looking statement can be guaranteed, and actual future results may vary materially. Factors that could cause actual results to differ from those discussed in the forward-looking statements include, but are not limited to:
• our strategies and objectives;
• our ability to continue to meet the Nasdaq requirements;
• our other financial operating objectives;
• the availability of qualified employees for business operations;
• general business and economic conditions;
• our ability to meet its financial obligations as they become due;
• the positive cash flows and financial viability of our operations and new business opportunities;
• our ability to manage growth with respect to our operations and new business opportunities;
• our ability to secure intellectual property rights over our proprietary products or enter into license agreements to secure the legal use of certain patents and intellectual property;
• our ability to avoid infringement of intellectual property rights; and
• our ability to be successful in new markets;
We describe certain material risks, uncertainties, and assumptions that could affect our business, including our financial condition and results of operations, under “Risk Factors.” We base our forward-looking statements on our management’s beliefs and assumptions based on information available to our management at the time the statements are made. We caution you that actual outcomes and results may, and are likely to, differ materially from what is expressed, implied or forecast by our forward-looking statements. Accordingly, you should be careful about relying on any forward-looking statements. Except as required under the federal securities laws, we do not have any intention or obligation to update publicly any forward-looking statements after the distribution of this prospectus, whether as a result of new information, future events, changes in assumptions, or otherwise.
16
THE LINCOLN PARK TRANSACTION
General
On March 13, 2025, we entered into the Purchase Agreement with Lincoln Park, which provides that, upon the terms and subject to the conditions and limitations set forth therein, we have the right, but not the obligation, to sell to Lincoln Park up to $12,000,000 of our Ordinary Shares, from time to time over the 24-month term beginning on the Commencement Date. Concurrently with entering into the Purchase Agreement, we also entered into the Registration Rights Agreement with Lincoln Park, pursuant to which we agreed to register the sale of our Ordinary Shares that have been and may be issued to Lincoln Park under the Purchase Agreement and that are subject to the offering described in this prospectus. Pursuant to the terms of the Purchase Agreement and Registration Rights Agreement, we have filed with the SEC this prospectus regarding the sale under the Securities Act of the shares that have been or may be issued to Lincoln Park under the Purchase Agreement.
Purchase of Shares under the Purchase Agreement
Regular Purchases
After the Commencement Date, on any business day selected by us, we may direct Lincoln Park to purchase up to 25,000 Ordinary Shares on such business day (or the purchase date), which we refer to as a Regular Purchase, provided that the closing sale price of our Ordinary Shares on Nasdaq is not below $0.25 on the applicable purchase date, and provided, further, that (i) a Regular Purchase shall be increased to up to 35,000 shares if the closing sale price of our Ordinary Shares on Nasdaq is not below $3.00 on the applicable purchase date and (ii) a Regular Purchase shall be increased to up to 50,000 shares if the closing sale price of our Ordinary Shares on Nasdaq is not below $4.00 on the applicable purchase date. However, we may not direct Lincoln Park to purchase more than $500,000 in shares under any single Regular Purchase.
The purchase price per share for each such Regular Purchase will be at a price equal to 95% of the lesser of:
• the lowest sale price for our Ordinary Shares on Nasdaq on the purchase date of such shares; and
• the average of the three lowest closing sale prices for our Ordinary Share on Nasdaq during the 10 consecutive business days immediately preceding the purchase date.
In addition, on the business day following the Commencement Date, which we refer to as the Effective Purchase Date, we have the option to sell to Lincoln Park in an effective purchase under the Purchase Agreement an amount of Ordinary Shares equal to $50,000 at a price per share calculated on the same basis as a Regular Purchase; provided, however, that such option will only be available on the Effective Purchase Date.
Accelerated Purchases
In addition, we may also direct Lincoln Park, on any business day on which we have submitted a Regular Purchase notice for the maximum amount allowed for such Regular Purchase, to purchase an additional amount of our Ordinary Shares, which we refer to as an Accelerated Purchase, of up to the lesser of:
(i) three times the number of shares purchased pursuant to such Regular Purchase; and
(ii) 30% of the trading volume of Ordinary Shares on Nasdaq during all or, if certain trading volume or market price thresholds specified in the Purchase Agreement are crossed on the applicable Accelerated Purchase date, the portion of the normal trading hours on the applicable Accelerated Purchase date prior to such time that any one of such thresholds is crossed, which period of time on the applicable Accelerated Purchase date we refer to as the Accelerated Purchase Period.
The purchase price per share for each such Accelerated Purchase will be equal to 95% of the lower of:
• the volume-weighted average price of our Ordinary Shares on Nasdaq during the applicable Accelerated Purchase Period on the applicable Accelerated Purchase date; and
• the closing sale price of our Ordinary Shares on Nasdaq on the applicable Accelerated Purchase date.
17
Additional Accelerated Purchases
We may also direct Lincoln Park on any business day on which an Accelerated Purchase has been completed and all of the shares to be purchased thereunder have been delivered to Lincoln Park in accordance with the Purchase Agreement, to purchase an additional amount of our Ordinary Shares, which we refer to as an Additional Accelerated Purchase, as described in the Purchase Agreement.
In the case of Regular Purchases, Accelerated Purchases and Additional Accelerated Purchases, the purchase price per share will be equitably adjusted for any reorganization, recapitalization, non-cash dividend, share split, reverse share split or other similar transaction occurring during the business days used to compute the purchase price.
The Purchase Agreement prohibits us from directing Lincoln Park to purchase any Ordinary Shares if those shares, when aggregated with all other Ordinary Shares then beneficially owned by Lincoln Park (as calculated pursuant to Section 13(d) of the Exchange Act and Rule 13d-3 thereunder), would result in Lincoln Park and its affiliates beneficially owning more than the Beneficial Ownership Limitation.
Pursuant to the terms of the Purchase Agreement, on March 13, 2025, we issued 175,000 Commitment Shares to Lincoln Park as consideration for its commitment to purchase our Ordinary Shares under the Purchase Agreement.
Although the Purchase Agreement provides that we may sell up to an aggregate of $12.0 million of our Ordinary Shares to Lincoln Park, only 4,000,000 Ordinary Shares are being registered for resale under this prospectus, which includes the 175,000 Commitment Shares that we already issued to Lincoln Park as a fee for making its irrevocable commitment to purchase our Ordinary Shares under the Purchase Agreement. Depending on the market prices of our Ordinary Shares at the time we elect to issue and sell our Ordinary Shares to Lincoln Park under the Purchase Agreement, we may need to register for resale under the Securities Act additional Ordinary Shares in order to receive aggregate gross proceeds equal to the $12.0 million total commitment available to us under the Purchase Agreement. If we elect to issue and sell to Lincoln Park under the Purchase Agreement more than the 3,825,000 Ordinary Shares being registered for resale by Lincoln Park under this prospectus, which we have the right, but not the obligation, to do, we must first register for resale under the Securities Act any such additional Ordinary Shares, which could cause additional substantial dilution to our shareholders. The number of Ordinary Shares ultimately offered for resale by Lincoln Park is dependent upon the number of Ordinary Shares we ultimately decide to sell to Lincoln Park under the Purchase Agreement.
To the extent we are subject to the applicable rules of Nasdaq, in no event may we issue or sell to Lincoln Park under the Purchase Agreement our Ordinary Shares, including the Commitment Shares, in excess of 1,881,328 shares, which is equal to 19.99% of our Ordinary Shares outstanding immediately prior to the execution of the Purchase Agreement, or the Exchange Cap, unless (i) we obtain shareholder approval to issue our Ordinary Shares in excess of the Exchange Cap or (ii) the average price of all Ordinary Shares issued to Lincoln Park under the Purchase Agreement equals or exceeds $1.29 per share (which represents the official closing price of our Ordinary Shares on Nasdaq the day of signing of the Purchase Agreement), such that the transactions contemplated by the Purchase Agreement are exempt from the Exchange Cap limitation under applicable Nasdaq rules. In any event, the Purchase Agreement specifically provides that we may not issue or sell any of our Ordinary Shares under the Purchase Agreement if such issuance or sale would breach any applicable rules or regulations of Nasdaq.
Sales under the Purchase Agreement may commence only after certain conditions have been satisfied, which date is referred to herein as the Commencement Date, which conditions include that this Registration Statement, as may be amended from time to time, shall have been declared effective under the Securities Act, delivery to Lincoln Park of a prospectus covering the Ordinary Shares issued or sold by us to Lincoln Park under the Purchase Agreement, approval for listing on the Nasdaq of the Ordinary Shares issued or sold by us to Lincoln Park under the Purchase Agreement, the issuance of the Commitment Shares to Lincoln Park under the Purchase Agreement, and the receipt by Lincoln Park of a customary opinion of counsel and other certificates and closing documents. The Purchase Agreement may be terminated by us at any time, at our sole discretion, without any cost or penalty, however, the Commitment Shares will not be returned to us. There are no limitations on use of proceeds, financial or business covenants, restrictions on future financings (other than restrictions on our ability to enter into additional “equity line” or similar transactions whereby an investor is irrevocably bound to purchase securities over a period of time from us at a price based on the market price of our Ordinary Shares at the time of such purchase), rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. We may deliver purchase notices under the Purchase Agreement,
18
subject to market conditions, and in light of our capital needs from time to time and under the limitations contained in the Purchase Agreement. Any proceeds that we receive under the Purchase Agreement may be used for any corporate purpose at our sole discretion.
The Company has agreed to file with the SEC, as soon as practicable, and in any event by May 15, 2025, this Registration Statement covering the resale of the Purchase Shares and the Commitment Shares in accordance with the terms of the Registration Rights Agreement.
The Purchase Agreement and Registration Rights Agreement contain customary representations and warranties, covenants and indemnification provisions that the parties made to, and solely for the benefit of, each other in the context of all of the terms and conditions of such agreements and in the context of the specific relationship between the parties thereto. The provisions of the Purchase Agreement and Registration Rights Agreement, including any representations and warranties contained therein, are not for the benefit of any party other than the parties thereto and are not intended as documents for investors and the public to obtain factual information about the current state of affairs of the parties thereto. Rather, investors and the public should look to other disclosures contained in our annual, quarterly and current reports we may file with the SEC.
Suspension Events
Suspension Events (the “Suspension Events”) under the Purchase Agreement include the following:
• the effectiveness of a Registration Statement registering the sale or resale of the Securities lapses for any reason (including, without limitation, the issuance of a stop order or similar order) or such registration statement (or the prospectus forming a part thereof) is unavailable to Lincoln Park for sale or resale of any or all of the Ordinary Shares to be issued to Lincoln Park under the Purchase Agreement and/or the Registration Rights Agreement, and all associated schedules and exhibits, that are required to be included therein, and such lapse or unavailability continues for a period of ten consecutive business days or for more than an aggregate of thirty business days in any 365-day period, but excluding a lapse or unavailability where (i) we terminate a registration statement after Lincoln Park has confirmed in writing that all of the Ordinary Shares covered thereby have been resold or (ii) we supersede one registration statement with another registration statement, including (without limitation) by terminating a prior registration statement when it is effectively replaced with a new registration statement covering the Ordinary Shares covered by the Purchase Agreement (provided in the case of this clause (ii) that all of the Ordinary Shares covered by the superseded (or terminated) registration statement that have not theretofore been resold are included in the superseding (or new) registration statement);
• the suspension of our Ordinary Shares from trading on the Nasdaq for a period of at least one business day, provided that we may not direct Lincoln Park to purchase any Ordinary Shares during any such suspension;
• the delisting of our Ordinary Shares from the Nasdaq Capital Market, provided, however, that our Ordinary Shares are not immediately thereafter trading on The Nasdaq Global Select Market, The Nasdaq Global Market, the New York Stock Exchange, the NYSE American, the NYSE Arca, or the OTCQB or the OTCQX operated by the OTC Markets Group, Inc. (or any nationally recognized successor to any of the foregoing);
• the failure for any reason by our transfer agent to issue Lincoln Park Ordinary Shares within two business days after the applicable date on which Lincoln Park is entitled to receive such shares;
• any breach of the representations or warranties or covenants contained in the Purchase Agreement or Registration Rights Agreement that has or could have a material adverse effect on us and, in the case of a breach of a covenant that is reasonably curable, that is not cured within five business days;
• if any person commences a proceeding against us pursuant to or within the meaning of any bankruptcy law;
• if we become at any time insolvent, or, pursuant to or within the meaning of any bankruptcy law, (i) commence a voluntary case, (ii) consent to the entry of an order for relief against us an involuntary case, (iii) consent to the appointment of a custodian for us or for all or substantially all of our property, or (iv) make a general assignment for the benefit of our creditors or if we are generally unable to pay our debts as the same become due;
19
• a court of competent jurisdiction enters an order or decree under any bankruptcy law that (i) is for relief against us in an involuntary case, (ii) appoints a custodian for us or for all or substantially all of our property, or (iii) orders the liquidation of us;
• if at any time we are not eligible to transfer Ordinary Shares electronically as DWAC Shares (as defined in the Purchase Agreement); or
• the Exchange Cap is reached (to the extent it is applicable) and our shareholders have not approved the issuance of Ordinary Shares pursuant to the Purchase Agreement, in accordance with the rules and regulations of Nasdaq.
Subject to the terms of the Purchase Agreement, so long as a Suspension Event has occurred and is continuing, or if any event that, after notice and/or lapse of time, would reasonably be expected to become a Suspension Event, has occurred and is continuing, we may not direct Lincoln Park to purchase any Ordinary Shares under the Purchase Agreement.
Our Termination Rights
We have the unconditional right, at any time, for any reason and without any payment or liability to us, to give one business day notice to Lincoln Park to terminate the Purchase Agreement.
No Short-Selling or Hedging by Lincoln Park
Lincoln Park has agreed that neither it nor any of its affiliates shall engage in any direct or indirect short-selling or hedging of Ordinary Shares during any time prior to the termination of the Purchase Agreement.
Prohibitions on Variable Rate Transactions
There are no restrictions on future financings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement or Registration Rights Agreement other than a prohibition on entering into a “Variable Rate Transaction,” as defined in the Purchase Agreement, for a period of twenty-four months following the date of the execution of the Purchase Agreement, other than with Lincoln Park. Variable Rate Transactions include an “equity line of credit” or any similar transaction whereby an investor is irrevocably bound to purchase securities over a period of time from us at a price based on the market price of our Ordinary Shares at the time of each such purchase, other than an “at the market offering” exclusively through a registered broker-dealer acting as our agent pursuant to a written agreement between us and such registered broker-dealer.
Effect of Performance of the Purchase Agreement on Our Shareholders
All shares registered in this offering that have been or may be issued or sold by us to Lincoln Park under the Purchase Agreement are expected to be freely tradable. It is anticipated that the Ordinary Shares registered in this offering will be sold over a period of up to twenty-four months commencing on the Commencement Date. The sale by Lincoln Park of a significant amount of Ordinary Shares registered in this offering at any given time could cause the market price of our Ordinary Shares to decline and to be highly volatile. Sales of our Ordinary Shares to Lincoln Park, if any, will depend upon market conditions and other factors to be determined by us. We may ultimately decide to sell to Lincoln Park all, some or none of the Ordinary Shares that may be available for us to sell pursuant to the Purchase Agreement.
If and when we do sell Ordinary Shares to Lincoln Park, after Lincoln Park has acquired the Ordinary Share, Lincoln Park may resell all, some or none of those Ordinary Shares at any time or from time to time in its discretion. Therefore, sales to Lincoln Park by us under the Purchase Agreement may result in substantial dilution to the interests of other holders of our Ordinary Shares. In addition, if we sell a substantial number of Ordinary Shares to Lincoln Park under the Purchase Agreement, or if investors expect that we will do so, the actual sales of our Ordinary Shares or the mere existence of our arrangement with Lincoln Park may make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect such sales. However, we have the right to control the timing and amount of any additional sales of our Ordinary Shares to Lincoln Park and the Purchase Agreement may be terminated by us at any time at our discretion without any cost to us.
20
Pursuant to the terms of the Purchase Agreement, from and after commencement, we have the right, but not the obligation, from time to time to direct Lincoln Park to purchase up to $12.0 million of our Ordinary Shares. Depending on the price per share at which we sell our Ordinary Shares to Lincoln Park pursuant to the Purchase Agreement, we may need to sell to Lincoln Park under the Purchase Agreement more Ordinary Shares than are being offered under this prospectus in order to receive aggregate gross proceeds equal to the $12.0 million total commitment available to us under the Purchase Agreement. If we choose to do so, we must first register for resale under the Securities Act such additional Ordinary Shares, which could cause additional substantial dilution to our shareholders. The number of Ordinary Shares ultimately offered for resale by Lincoln Park under this prospectus is dependent upon the number of Ordinary Shares we direct Lincoln Park to purchase under the Purchase Agreement.
The following table sets forth the amount of gross proceeds we would receive from Lincoln Park from our sale of Ordinary Shares to Lincoln Park under the Purchase Agreement at varying purchase prices:
|
Assumed Average Purchase Price Per Share |
Number of |
Percentage of |
Gross Proceeds |
||||||
|
$0.50 |
1,706,328 |
(3) |
16.03 |
% |
$ |
853,164 |
|||
|
$1.00 |
1,706,328 |
(3) |
16.03 |
% |
$ |
1,706,328 |
|||
|
$1.29(4) |
1,706,328 |
(3) |
16.03 |
% |
$ |
2,201,163 |
|||
|
$2.00 |
3,825,000 |
|
28.87 |
% |
$ |
7,650,000 |
|||
|
$3.00 |
3,825,000 |
|
28.87 |
% |
$ |
11,475,000 |
|||
____________
(1) Although the Purchase Agreement provides that we may sell up to $12.0 million of our Ordinary Shares to Lincoln Park, we are only registering 4,000,000 of our Ordinary Shares for resale under this prospectus, including 175,000 Commitment Shares that we have already issued to Lincoln Park as a fee for making its irrevocable commitment to purchase our Ordinary Shares under the Purchase Agreement. Depending on the market prices of our Ordinary Shares at the time we elect to issue and sell our Ordinary Shares to Lincoln Park under the Purchase Agreement, we may need to register for resale under the Securities Act additional Ordinary Shares in order to receive aggregate gross proceeds equal to the $12.0 million total commitment available to us under the Purchase Agreement.
(2) The denominator is based on 10,028,025 shares outstanding as of June 27, 2025, which includes the Commitment Shares, and adjusted to include the issuance of the number of shares set forth in the adjacent column that we would have sold to Lincoln Park, assuming the average purchase price in the first column. The numerator reflects the number of shares set forth in the adjacent column which we would have sold under the Purchase Agreement at the corresponding assumed average purchase price set forth in the adjacent column, and the Commitment Shares.
(3) This number of shares reflects the Exchange Cap, less the Commitment Shares. To the extent we are subject to the applicable Nasdaq rules, we may only issue Ordinary Shares in excess of the Exchange Cap if we obtain shareholder approval to do so, or if the average price of all Ordinary Shares issued to Lincoln Park under the Purchase Agreement equals or exceeds $1.29 per share.
(4) The closing sale price of our Ordinary Shares on The Nasdaq Capital Market on March 13, 2025.
21
USE OF PROCEEDS
We will not receive any proceeds from the sale of Ordinary Shares by Lincoln Park in this offering. We may receive up to $12,000,000 in aggregate gross proceeds under the Purchase Agreement from any sales we make to Lincoln Park from time to time pursuant to the Purchase Agreement after the date that the registration statement of which this prospectus is a part is declared effective. The gross proceeds to us from the sale of Ordinary Shares to Lincoln Park pursuant to the Purchase Agreement will be up to $12,000,000 over an approximately 24-month period, assuming that such sales are not limited by the Exchange Cap and we sell the full amount of Ordinary Shares that we have the right, but not the obligation, to sell to Lincoln Park under the Purchase Agreement, and before other estimated fees and expenses. We may sell fewer than all of the Ordinary Shares permitted to be sold pursuant to the Purchase Agreement, in which case our offering proceeds will be less. Because we are not obligated to issue or sell any Ordinary Shares under the Purchase Agreement, other than the Commitment Shares (from which we did not receive any proceeds) or at our election, the Effective Purchase Shares (from which we may receive proceeds), the actual total offering amount and proceeds to us, if any, are not determinable at this time. There can be no assurance that we will receive any proceeds under or fully utilize the Purchase Agreement. See “Plan of Distribution” elsewhere in this prospectus for more information.
As of the date of this prospectus, we cannot predict with certainty all the uses for the net proceeds, if any, we may receive from the sale of Purchase Shares to Lincoln Park under the Purchase Agreement. We currently intend to use such net proceeds, if any, for the Company’s research and development, marketing activities and general working capital.
Changing circumstances may cause us to consume capital significantly faster than we currently anticipate. The amounts and timing of our actual expenditures will depend upon numerous factors, including the progress of our global marketing and sales efforts, our development efforts and the overall economic environment. Therefore, our management will retain broad discretion over the use of the proceeds from this offering. We may ultimately use the proceeds for different purposes than what we currently intend. Pending any ultimate use of any portion of the proceeds from this offering, if the anticipated proceeds will not be sufficient to fund all the proposed purposes, our management will determine the order of priority for using the proceeds, as well as the amount and sources of other funds needed.
22
DILUTION
The sale of Ordinary Shares to Lincoln Park pursuant to the Purchase Agreement will have a dilutive impact on our shareholders. In addition, the lower the price of our Ordinary Shares is at the time we exercise our right to sell shares to Lincoln Park, the more Ordinary Shares we will issue to raise our desired amount of proceeds from the sale, and the greater the dilution to our existing shareholders.
The price that Lincoln Park will pay for our Ordinary Shares to be resold pursuant to this prospectus will depend upon the timing of sales and will fluctuate based on the trading price of the Ordinary Shares.
The historical net tangible book value (deficit) of our Ordinary Shares was approximately $(764,540), or $(0.079) per share as of December 31, 2024. Historical net tangible book value (deficit) per share represents the amount of our total tangible assets less our total liabilities, divided by 9,651,102, the total number of Ordinary Shares issued and outstanding on December 31, 2024.
The pro forma historical net tangible book value was approximately $(230,120) at December 31, 2024. Pro forma historical net tangible book value per share represents the amount of our total tangible assets less our total liabilities, divided by the total number of Ordinary Shares outstanding at December 31, 2024, after giving effect to (i) the issuance of 201,923 Ordinary issuable upon conversion of promissory notes outstanding as of December 31, 2024 at a weighted average conversion price of USD$2.14 per Ordinary Share.
After giving effect to (i) the sale of 3,825,000 Purchase Shares to Lincoln Park pursuant to the Purchase Agreement, which reflects the number of shares that we have registered and we may issue to Lincoln Park under this prospectus, and assuming gross proceeds of approximately $4.93 million from the sale of shares to Lincoln Park pursuant to the Purchase Agreement (based on an assumed price of $1.29 per share, the closing price of our Ordinary Shares on The Nasdaq Capital Market on March 13, 2025), which assumes no application of the Exchange Cap, (ii) the issuance of 175,000 Ordinary Shares as Commitment Shares, and (iii) deducting estimated offering expenses of $182,000 payable by us, our pro forma net tangible book value as of December 31, 2024, would have been approximately $4.5 million, or $0.326 per share. This represents an immediate increase in net tangible book value of $0.349 per share and an immediate dilution of $(0.964) per share to new investors.
The following table illustrates this dilution on a per share basis:
|
Assumed offering price per share |
$ |
1.29 |
|
|
|
Historical net tangible book deficit per Ordinary Share at December 31, 2024 |
$ |
(0.079 |
) |
|
|
As adjusted net tangible book value per Ordinary Share as December 31, 2024, after giving effect to the pro forma transactions |
$ |
(0.023 |
) |
|
|
As adjusted net tangible book value per share as of December 31, 2024, after giving effect to the offering attributable to new investors |
$ |
0.326 |
|
|
|
Dilution per Ordinary Share to new investors in this offering |
$ |
(0.964 |
) |
The table above excludes the following as of December 31, 2024:
• 91,000 of our Ordinary Shares issuable upon the exercise of underwriter’s warrants issued to the Benchmark Company as representative of the underwriters for our IPO, outstanding as of December 31, 2024 at an exercise price of $5.00 per Ordinary Share.
23
MANAGEMENT DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of the financial condition and results of our operations should be read in conjunction with the “Summary Statements of Operations Data” and our consolidated financial statements and the notes to those statements appearing elsewhere in this prospectus. This discussion and analysis contains forward-looking statements reflecting our management’s current expectations that involve risks, uncertainties and assumptions. Our actual results and the timing of events may differ materially from those described in or implied by these forward-looking statements due to a number of factors, including those discussed below and elsewhere in this prospectus particularly in the Section entitled “Risk Factors”.
Overview
Our company was incorporated as a proprietary company limited by shares under the laws of Australia in October 2018. The name of the Company was changed from Myhypo Pty Ltd to Gelteq Pty Ltd in connection with the expansion of the business across a wider set of markets and became Gelteq Limited upon conversion into a public company on May 26, 2022. We are engaged in the research development and testing of a gel based delivery system for humans and pets. The registered office of the company is Vistra Australia, Level 4, 100 Albert Road, South Melbourne VIC, 3025 Australia. Our principal place of business is Monash Innovation Labs, G. 60, 22 Alliance Lane, Clayton 3800, Victoria, Australia and our telephone number is +61 3 9087 3990. See “Description of Share Capital and Constitution.”
Business Overview
We are a clinical and science-based company that is focused on developing and commercializing white label gel-based delivery solutions for prescription drugs, nutraceuticals, pet care and other products. A “white label” gel-based delivery solution is where we produce a product that other companies rebrand as their own product. Our principal products are edible gels, which we refer to as gels, and their application in gel-based dosage forms. Our current product suite consists of multiple products that sit within five core verticals — for pets, sports, pharmaceutical (pharma), over-the-counter (OTC) and nutraceutical — all of which leverage our patent pending multiple-ingredient dosage forms, and that we expect to have a wide range of applications and consumers. We currently focus our efforts on out-licensing our technology to companies to develop and create new products they can manufacture and sell within their established and researched markets, while we continue to manufacture our existing products under license (“white label”).
Of our products already licensed, two clients have placed initial orders for nutraceutical products, and there have been four other products in the sports vertical ordered. From these orders, we shipped 15,000 units during May 2022, 250,000 units during June 2022 and 60,000 units in December 2022. For the year ended June 30, 2023, the 60,000 units delivered in December 2022 has been recognized as revenue of AUD$79,843 (USD$51,898) from the deferred revenue balance at June 30, 2022. The Company has shipped the remaining orders in the fiscal year ending June 30, 2025. In January 2023, one of our existing clients placed further orders for two new products totaling 120,000 units, of which we received a AUD$45,437 (USD$29,534) non-refundable deposit for such orders in May 2023, and a new client placed an order for 80,000 units. In October 2023, we received a further order for 200,000 units in our nutraceutical vertical, of which we received a non-refundable deposit of AUD$40,000 (USD$26,000). We have shipped such orders in the fiscal year ending June 30, 2025 as our clients have resolved their cash flow issues. We have also put in place greater rigorous qualification procedures to ensure future customers have the financial ability to fund orders through manufacturing in a timely manner.
With regards to the pets, nutraceutical and sports vertical, we designed these products to have no regulatory hurdles to overcome as they have food grade classifications and therefore do not require regulatory approvals. We designed our gel platform to enhance the tolerability and stability of drugs while maintaining their efficacy. Products in the pharma vertical will require regulatory approval.
Financial Operations Overview
Revenues
For the year ended June 30, 2023, we delivered 60,000 units in December, 2022 and recognized revenue of AUD$79,843 (USD$51,898) from the deferred revenue balance at June 30, 2022. The delay in completing the manufacturing in
24
support of the new January 2023 orders, and being able to recognize the relevant component of the existing deferred revenue balance as income, is due to certain of our customers that experienced cash flow difficulties and therefore being unable to pay for the order at the time.
For the six months ended December 31, 2024, we had prioritized pharmaceutical research and improving operational processes, and we expect to grow and execute on our business plans with lower overheads and expenses in the financial year ending June 30, 2025. To facilitate this, we entered into a rental contract, filed as an exhibit to the registration statement of which this prospectus forms a part, for laboratory facilities with Monash University on February 2, 2024 (the “Monash Facilities”) for further research purposes. Our lack of personnel, and our focus on research, and identifying and establishing a laboratory facility, adversely affected our ability to close new sales opportunities. We believe this will have a short-term impact on sales revenue which was nil for the six months ended December 31, 2023.
With the Monash Facilities established and fitted, as well as the closing of our IPO, we are prioritizing our sales activities with a focus on the animal health, nutraceutical, sports, over-the-counter and pharmaceuticals verticals. Notwithstanding the foregoing we are currently prioritizing pharmaceutical research on our existing 505b(2) application and seeking other potential pharmaceutical candidates through such pathway.
We continue to discuss revenue opportunities with existing and prospective customers and we remain confident in our sales strategy and our strong existing new business pipeline, and we would fulfil our revenue numbers should each existing potential client in the pipeline eventuate. However, for the business to generate its expected revenue from products sales and licenses in the financial year ending June 30, 2026, we need to ensure the following events will occur:
1) Manufacturing — As we continue to have part of our manufacturing process in Xiamen, Fujian, China, we remain confident that products will still be manufactured and shipped to our customers globally. However, given the follow-on effects to the Chinese economy due to stringent protocols of COVID-19 there and together with emerging cross-border tariffs that impact the cost of goods, supply chains and pricing, we must remain vigilant on any potential change. We also rely on all raw materials being readily available both in China and in our US operations. We are continuing to see first-hand delays of ingredients reaching our manufacturers on time.
2) Advertising — We have allowed for a substantial advertising budget in the financial year ending June 30, 2026 to introduce the business and our products and services to potential licensees. This will include a combination of increased sales staff, attendance at relevant exhibitions and conferences, and more traditional online advertising and marketing efforts. The business will also be launching a series of mini websites, each site based on our products, to educate and serve as a resource material to our existing customers and potential customers. This would in turn potentially sell Gelteq products and to initiate more relevant marketing activity.
3) Existing Clients — We already have existing licensees. Many of our clients have forecast future orders later this calendar year, and we believe these orders will assist us in realizing our desired revenue targets. At the date of this prospectus, we expect approximately 1 million units to be ordered from existing customers, with many of these being treated as pilots with lower margins. We anticipate that such orders would increase our products’ market exposure in the wider market; additional orders from these clients may provide increased sales revenues and gross margins. In addition, we would be in a position to negotiate higher per unit pricing for any new clients we acquire subsequent to the pilot sales, which in turn would provide higher overall margins for the business. As such, we thereby believe that the initial sales may generate the conditions for further revenues which would improve our financial position. However, it is the additional revenue opportunities that may develop as a result of these orders, and which are not immediately quantifiable, that we believe will provide a potential revenue source during the year ended June 30, 2026. As part of our sales effort, we have engaged a sales and marketing firm in the Asia Pacific region to help launch our sporting products on our new online stores in China and we expect such stores to provide additional revenue during the year ended June 30, 2026. There is no guarantee that all or any of pre-ordered amounts will come to fruition, as we depend on our customers’ cash flows to manufacture
25
the products as well as the outcome of the initial trial orders for some of our licensees. Our customers that had cash flow difficulties had resolved them, and we have shipped these orders in the fiscal year ending June 30, 2025.
4) New Hires — To date, we have not been adequately staffed to be able to reach our projected forecasted revenues. We have been focused on selecting the right new hires to directly assist us to reach our revenue targets, with these hires to be spread across the business to ensure all sectors are adequately staffed and working towards business performance. We expect that we will onboard an additional three sales managers in the year ending June 30, 2026 once adequate funds have been raised to assist us in meeting our revenue targets.
Operating expenses — for the six months ended December 31, 2024
Our company’s focus has been on research, with our operating expenses being made up of corporate and administrative expenses together with research expenses.
Research expenses — for the six months ended December 31, 2024
Our research expenses consist of:
• salaries for research staff and consultants, including employee benefits;
• expenses paid to contracted University for product testing, validation and pre-clinical studies; and
• raw material expenses.
The primary research on our gel based delivery system is complete and the Company has already begun manufacturing across different product verticals in May 2022.
With our product verticals, in the financial year ending June 30, 2026, we will prioritize research and development in our pharmaceutical/OTC vertical. Unlike foods, nutraceuticals, and sporting verticals, pharmaceutical and OTC regulations are stricter and require clinical work or studies. Clinical research and development costs differ at different stages of the product research and development cycle. As our focus is on the 505(b)(2) pathway, these expenses are substantially less than that of a new drug development. However, the studies required can still be unpredictable in cost. While we do all the required lab work possible prior, there is inherent uncertainly in a clinical trial that makes it difficult to be assured of the time when the results will arrive and whether additional trials are needed. Given this, the timing for income generation from these products has uncertainties and we may require additional research and development costs to finalize a product.
The 505(b)(2) pathway is the shortest timeline we can take to register a product with the FDA as the approved timeline requires stability and bioequivalence data rather than three phases of clinical trials. Any trials which have a negative outcome, or any requirements from a regulatory body for additional data will create a delay to income and increase our research and development costs which in turn can have a material adverse effect on our operations.
Corporate and administrative expenses — for the six months ended December 31, 2024
Our corporate and administrative expenses are primarily made up of staff and consultants’ salaries, employee benefits, professional fees for auditors, consultants and legal counsel and advertising and marketing expenses. Such expenses are incurred in the process of becoming an Australian public company that is to be treated as a public company in the United States.
We can expect the corporate and administrative expenses to increase through an increase in staffing expenses and employee benefits, legal and auditor professional fees, fees associated with stock exchange listing and SEC requirements, investor relations expenses and insurances.
As we have products ready for commercialization, the increase in staff expenses is expected to prepare for commercial operations, in particular around sales and marketing of our products.
26
Financial expenses — for the six months ended December 31, 2024
Financial expenses mainly includes interest on existing shareholders’ loans at an interest rate of 12% per annum, with a term of 18 months and maturing on July 15, 2023. On January 3, 2023, the existing shareholder loans the Company entered into on January 20, 2022 (totaling AUD$1,493,445 at an interest rate of 12% per annum maturing on July 15, 2023) were extended for an additional 12 months at an interest rate of 12% per annum maturing on July 15, 2024. The foregoing loan extension constitutes a substantial modification per IFRS 9, and therefore the original liability is derecognized on modification date, and the new liability for the extended loans is recognized at fair value discounted using an appropriate discount rate. The resulting gain on the modification of the liability (AUD$222,681, USD$144,743) is recognized in the consolidated statements of profit or loss and other comprehensive income in the June 30, 2023 financial statements. For the six month period ended December 31, 2024, the Company incurred financial expenses of approximately AUD$637,594 (USD $414,436) from the closing of its IPO and costs incurred in connection with its continued listing on Nasdaq (December 31, 2023: AUD$102,941). Also, as products are manufactured and sold, together with necessary clinical trials, we can expect an increase in financial expenses which will consist mainly of expenses related to foreign currency exchange transactions and standard bank charges.
Historical Financial Performance — For the six month period ended December 31, 2024 and year ended June 30, 2024 compared to the six month period ended December 31, 2023 and the year ended June 30, 2023.
The Company presents and reports its financial statements in accordance with International Financial Reporting Standards (IFRS) and in Australian Dollars (AUD$ or A$), its presentation currency.
Historical information
The Company’s financial statements for the year ended June 30, 2024 and period ended December 31, 2024 have been audited and reviewed by M&K CPAS, PLLC respectively and the Company’s financial statements for the year ended June 30, 2023 have been audited by UHY Haines Norton, Sydney in accordance with the standards of the Public Company Accounting Oversight Board (“PCAOB”). Management’s discussion and analysis of our financial position and results of operations is based on our consolidated financial statements, which we have prepared in accordance with International Financial Reporting Standards and International Accounting Standards as issued by the International Accounting Standards Board (IASB) and Interpretations (collectively IFRSs).
The preparation of the financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results.
27
Financial Position in AUD$:
|
As at |
As at |
As at |
As at |
||||||||
|
ASSETS |
|
|
|
||||||||
|
Current Assets |
|
|
|
||||||||
|
Cash and cash equivalents |
24,522 |
399,224 |
|
3,046,602 |
|
24,522 |
|
||||
|
Trade and other receivables |
183,004 |
345,291 |
|
305,007 |
|
183,004 |
|
||||
|
Inventories |
— |
95,201 |
|
— |
|
— |
|
||||
|
Prepayments and other assets |
95,700 |
151,258 |
|
1,587,521 |
|
95,700 |
|
||||
|
Total Current Assets |
303,227 |
990,974 |
|
4,939,130 |
|
303,227 |
|
||||
|
|
|
|
|||||||||
|
Non-Current Assets |
|
|
|
||||||||
|
Plant and equipment |
16,642 |
— |
|
18,056 |
|
16,642 |
|
||||
|
Right-of-use assets |
— |
10,001 |
|
— |
|
— |
|
||||
|
Intangible Assets |
20,437,958 |
21,493,661 |
|
20,158,270 |
|
20,437,958 |
|
||||
|
Total Non-Current Assets |
20,454,600 |
21,503,662 |
|
20,176,326 |
|
20,454,600 |
|
||||
|
Total Assets |
20,757,827 |
22,494,636 |
|
25,115,456 |
|
20,757,827 |
|
||||
|
|
|
|
|||||||||
|
LIABILITIES |
|
|
|
||||||||
|
Current Liabilities |
|
|
|
||||||||
|
Trade and other payables |
1,558,186 |
1,184,404 |
|
892,791 |
|
1,558,186 |
|
||||
|
Deferred Revenue |
125,359 |
85,359 |
|
118,704 |
|
125,359 |
|
||||
|
Borrowings, net |
2,084,152 |
5,086 |
|
3,882,778 |
|
2,084,152 |
|
||||
|
Derivative liability |
— |
— |
|
1,279,184 |
|
— |
|
||||
|
Lease liabilities |
— |
11,896 |
|
— |
|
— |
|
||||
|
Employee benefit provisions |
98,368 |
77,780 |
|
105,198 |
|
98,368 |
|
||||
|
Total Current Liabilities |
3,866,065 |
1,364,525 |
|
6,278,655 |
|
3,866,065 |
|
||||
|
|
|
|
|||||||||
|
Non-Current Liabilities |
|
|
|
||||||||
|
Borrowings |
1,759,447 |
2,471,619 |
|
13,550 |
|
1,759,447 |
|
||||
|
Lease liabilities |
— |
— |
|
|
|
— |
|
||||
|
Employee benefit provisions |
20,018 |
— |
|
29,488 |
|
20,018 |
|
||||
|
Total Non-Current Liabilities |
1,779,465 |
2,471,619 |
|
43,038 |
|
1,779,465 |
|
||||
|
Total Liabilities |
5,645,530 |
3,836,144 |
|
6,321,693 |
|
5,645,530 |
|
||||
|
Net Assets |
15,112,297 |
18,658,492 |
|
18,793,763 |
|
15,112,297 |
|
||||
|
|
|
|
|||||||||
|
EQUITY |
|
|
|
||||||||
|
Issued capital |
26,608,227 |
26,608,227 |
|
33,594,052 |
|
26,608,227 |
|
||||
|
Reserves |
|
— |
|
|
|
|
|
||||
|
Accumulated losses |
(11,495,930) |
(7,949,735 |
) |
(14,800,289 |
) |
(11,495,930 |
) |
||||
|
Total Equity (Deficit) |
15,112,297 |
18,658,492 |
|
18,793,763 |
|
15,112,297 |
|
||||
28
Years ended June 30, 2024 and 2023
Extract of Statement of comprehensive income (in AUD$)
The following table summarizes the results of operations for the years ended June 30, 2024 and 2023:
|
Year ended June 30 |
||||||
|
2024 |
2023 |
|||||
|
AUD$ |
AUD$ |
|||||
|
Revenue from contract with customers |
— |
|
79,843 |
|
||
|
Raw materials and consumable expenses |
— |
|
(48,925 |
) |
||
|
Research expenses |
(276,057 |
) |
(665,035 |
) |
||
|
Corporate & administrative expenses |
(3,417,022 |
) |
(3,412,672 |
) |
||
|
Gains from loan modification |
— |
|
222,681 |
|
||
|
Other income |
146,884 |
|
317,888 |
|
||
|
Loss before income tax |
(3,546,195 |
) |
(3,506,220 |
) |
||
|
Income tax expense |
— |
|
— |
|
||
|
Loss after income tax |
(3,546,195 |
) |
(3,506,220 |
) |
||
Revenue from contract with customers
During the year ended June 30, 2024, revenue from contract with customers decreased by AUD$79,843 to nil (June 30, 2023 AUD$79,843). This decrease is attributable to undelivered orders as some of the Company’s customers experienced cashflow difficulties and were unable to pay for their outstanding orders. However, such customers have resolved such issues and we have shipped their orders in the fiscal year ending June 30, 2025.
Raw materials and consumable expenses
No costs have been incurred on raw material and consumables expenses during the year ended June 30, 2024.
During the year ended June 30, 2023, the Company incurred raw materials and consumables expenses of AUD$48,925. The Company incurred these expenses to help facilitate the manufacturing of products with its contract manufacturers. The reduction in expenses is due to less products being manufactured in this period.
Research expenses
During the year ended June 30, 2024, research expenses decreased by approximately 58% (AUD$388,978) to AUD$276,057 as compared to the similar period last year (2023: AUD$665,035). The decrease in research expenses is due to less product testing and validations conducted and greater time spent to setup new research laboratory facilities. The lower volume of product testing and validations reduced the amount of external costs borne by the Company, which resulted in lower research costs. Research expenses are those focused primarily on research projects, and as such the Company does not allocate employee involvement in establishing research facilities to research expenses. Therefore, employee involvement in establishing the new research facilities also led to lower research expenditures for the period ended June 30, 2024 compared to June 30, 2023.
Cash and cash equivalents
Cash and cash equivalents decreased by AUD$374,702 to AUD$24,522 at June 30, 2024 as compared to June 30, 2023, of AUD$399,224, as a result of decrease in cash inflow from financing activities and increase in cash used in investing activities offset by decrease in cash used in operating activities.
For the year ended June 30, 2024, net cash used in operating activities decreased by AUD$699,964 to AUD$1,070,471 relative to AUD$1,770,435 for the corresponding period last year. The decrease is primarily attributable to a decrease in payments to suppliers and employees of AUD$505,278 (June 30, 2024, AUD$1,372,801 compared to AUD$1,878,079 at June 30, 2023) and payment to initial public offering suppliers of AUD$160,489 (June 30, 2024, Nil compared to AUD$160,489 at June 30, 2023).
The decrease in cash used in operating activities was offset by increase in cash used in investing activities of AUD$72,239 due to acquisition of intangible assets and property, plant and equipment.
29
The decrease in cash used in operating activities was also offset by decrease in cash from financing activities attributable to nil proceeds from share issuance. (June 30, 2023: AUD$1,431,162)
Trade and other receivables
Trade and other receivables decreased by AUD$162,286 to AUD$183,005 at June 30, 2024 as compared to AUD$345,291 as at June 30, 2023. The decrease was predominantly due to a decrease in the research and development tax refund receivable of AUD$119,744.
Machinery acquisition costs increased by AUD$16,642 as at June 30, 2024 relative to nil at June 30, 2023 due to acquisition of property, plant and equipment.
Inventories
Inventories decreased by AUD$95,201 to nil as at June 30, 2024 due to an inventory write off (June 30, 2023: AUD$95,201).
Intangible Assets
Intangible assets (including right-of-use assets) decreased by AUD$1,065,704 to AUD$20,437,958 at June 30, 2024 as compared to AUD$21,503,662 as at June 30, 2023, predominantly due to amortization of AUD$1,210,724 for the year ended June 30, 2024 offset by increase in patents and trademarks of AUD$145,020.
Trade and Other payables
Trade and other payables increased by AUD$373,782 to AUD$1,558,186 at June 30, 2024 as compared to AUD$1,184,404 as at June 30, 2023, primarily attributable to an increase in Pay As You Go (PAYG) withholding payable of AUD$265,082 to AUD$314,599 (June 30, 2023: AUD$49,517) and an increase in superannuation payable of AUD$104,739 to AUD$121,530 (June 30, 2023: AUD$16,791).
Gains from loan modification
During the year ended June 30, 2024, gains from loan modification decreased by AUD$222,681 to nil at as there was no modification of loan (June 30, 2023: AUD$222,681).
Other Income
Other income for the year ended June 30, 2024 has decreased by AUD$171,004 to AUD$146,884 as compared to AUD$317,888 for the year ended June 30, 2023. Other income comprises the Research and Development tax incentive and foreign exchange gain.
The Company is eligible for the Australian Government Research and Development Tax Incentive (“R&D Tax Incentive”) that provides tax offsets for expenditure on eligible R&D activities. Under the program, the Company is entitled to a refundable R&D credit in Australia on the eligible R&D expenditure incurred on eligible R&D activities. The R&D Tax Incentive is overseen by the Australian Taxation Office and AusIndustry, a business advisory arm of the Australian government. The R&D Tax Incentive legislation, Income Tax Assessment Act 1997, Division 355, provides for a refundable R&D tax offset equal to the Company’s corporate tax rate plus an 18.5% premium for companies with an aggregated turnover of below AUD$20 million.
The refundable R&D tax offset is accounted for under IAS 20 Accounting for Government Grants and Disclosure of Government Assistance, as per which the R&D tax offset income is recognized when there is reasonable assurance that it will be received. It is recognized in the statement of comprehensive income in the same period that the related costs are recognized as expenses and relates to refundable amounts on approved expenses.
Deferred revenue
Deferred revenue as at June 30, 2024 stands at AUD$125,359 as compared to AUD$85,359 at June 30, 2023 reflecting an increase of AUD$40,000. Deferred revenue represents amounts received for purchase orders that are yet to be delivered as at June 30, 2024.
30
Borrowings (current and non-current)
Borrowings at June 30, 2024, stands at AUD$3,843,599 representing: loans of AUD$18,636 received from Directors of which AUD$5,086 is current and AUD$13,550 is non-current; shareholder loan of AUD$1,923,000 (current), convertible notes of AUD$1,745,897 (non-current) and loans from associated entities of AUD$156,066 (current). Borrowings during the June 30, 2024 financial year had increased to AUD$3,843,599 as compared to AUD$2,476,705 for the year ended June 30, 2023, due to the issuance of new convertible notes, which stands at AUD$1,745,897 as at June 30, 2024 (June 30, 2023: AUD$839,115), and issuance of new shareholder loan which stands at AUD$1,923,000 as at June 30, 2024 (June 30, 2023: AUD$1,463,650).
Corporate and administrative expenses (in AUD)
|
Year ended |
||||
|
2024 |
2023 |
|||
|
AUD$ |
AUD$ |
|||
|
Employment expenses |
875,579 |
752,584 |
||
|
Corporate expenses |
222,641 |
428,922 |
||
|
IPO related expenses |
166,084 |
278,319 |
||
|
Depreciation and amortization expenses |
1,211,896 |
1,226,491 |
||
|
Advertising & marketing expense |
18,200 |
166,929 |
||
|
Share based expense |
— |
— |
||
|
Intellectual Property Services |
— |
— |
||
|
Legal expenses |
— |
5,270 |
||
|
Consulting fees |
750 |
80,407 |
||
|
Other expenses |
145,851 |
69,681 |
||
|
Finance costs |
600,220 |
404,069 |
||
|
Total Corporate and administrative expenses |
3,241,221 |
3,412,672 |
||
During the year ended June 30, 2024, total corporate and administrative expenses decreased by AUD$171,451 to AUD$3,241,221 relative to AUD$3,412,672 in the similar period last year.
The AUD$171,451 decrease in corporate and administrative expenses during the year ended June 30, 2024, relative to June 30, 2023, was predominantly due to decrease in (i) corporate expenses of AUD$206,281 due to lower professional and management fees; (ii) advertising and marketing expenses of AUD$148,729 due to fewer marketing activities; and (iii) consulting fees of AUD$79,657 due to a decrease in external consultants used during the year. The decrease in corporate and administrative expenses was offset by an increase in (i) finance cost of AUD$196,151 due to additional interest relating to the shareholders loans and convertible notes and (ii) employment expenses of AUD$122,995 attributable to an increase in permanent and contract staff.
For the six months ended December 31, 2024 and 2023
Extract of Statement of comprehensive income (in AUD$)
The following table summarizes the results of operations for the six months ended December 31, 2024 and 2023:
|
Six months ended |
||||||
|
2024 |
2023 |
|||||
|
AUD$ |
AUD$ |
|||||
|
Revenue from contract with customers |
— |
|
— |
|
||
|
Raw materials and consumable expenses |
— |
|
— |
|
||
|
Research expenses |
(314,472 |
) |
(100,934 |
) |
||
|
Corporate & administrative expenses |
(3,301,299 |
) |
(1,661,589 |
) |
||
|
|
|
|||||
|
Other income |
311,412 |
|
76,879 |
|
||
|
Loss before income tax |
(3,304,359 |
) |
(1,685,644 |
) |
||
|
Income tax expense |
|
|
||||
|
Loss after income tax |
(3,304,359 |
) |
(1,685,644 |
) |
||
31
Revenue from contracts with customers
During the six months ended December 31, 2024 and 2023, revenue from contracts with customers was $Nil for both periods.
Raw materials and consumable expenses
During the six months ended December 31, 2024 and 2023, the Company did not incur any raw materials and consumables expenses.
Research expenses
During the six months ended December 31, 2024, research expenses increased by approximately 212% (AUD$213,538) to AUD$314,472 as compared to the six months ended December 31, 2023 (AUD$100,934). The increase in research expenses is due to increased product testing and validations conducted and more time diverted to setting up new research laboratory facilities.
Cash and cash equivalents
Cash and cash equivalents increased by AUD$3,022,080 to AUD$3,046,602 at December 31, 2024 as compared to June 30, 2024, AUD$24,522, as a result of net cash from financing activities of AUD$6,798,332 (June 30,2024: AUD$843,939) attributable to proceeds from issue of shares from the Company’s IPO (net of capital issue costs) and borrowings proceeds offset by increase in cash used in operating activities of AUD$ 3,594,665 (June 30, 2024: AUD$1,070,471) attributable to increase in payments to suppliers and employees, as well as offset by increase in cash used in investing activities of AUD$328,222 (June 30, 2024: AUD$148,170) attributable to increase in payment for acquisition of intangibles and property, plant and equipment.
Trade and other receivables
Trade and other receivables increased by AUD$122,002 to AUD$305,007 at December 31, 2024 as compared to AUD$183,005 as at June 30, 2024.
Intangible Assets
Intangible assets (including right-of-use assets) decreased by AUD$279,688 to AUD$20,158,270 at December 31, 2024 as compared to AUD$20,437,958 as at June 30, 2024, predominantly due to amortization of AUD$606,497 of trade secrets for the period ended December 31, 2024.
Trade and Other payables
Trade and other payables decreased by AUD$665,395 to AUD$892,791 at December 31, 2024 as compared to AUD$1,558,186 as at June 30, 2024, primarily attributable to the repayment of long outstanding salary payable, PAYG and superannuation.
Other Income
Other income for the period ended December 31, 2024 has increased by AUD$234,533 to AUD$311,412 as compared to AUD$76,879 for the period ended December 31, 2023. Other income comprises the Research and Development tax incentive and foreign exchange gain discussed below. The Company has received in the period ended December 31, 2024 the R&D incentive for the period ended June 30, 2023 which explains the increase as compared to the period ended December 31, 2023.
The Company is eligible for the Australian Government Research and Development Tax Incentive (“R&D Tax Incentive”) that provides tax offsets for expenditure on eligible R&D activities. Under the program, the Company is entitled to a refundable R&D credit in Australia on the eligible R&D expenditures incurred on eligible R&D activities. The R&D Tax Incentive is overseen by the Australian Taxation Office and AusIndustry, a business advisory arm of the Australian government. The R&D Tax Incentive legislation, Income Tax Assessment Act 1997, Division 355, provides for a refundable R&D tax offset equal to the Company’s corporate tax rate plus an 18.5% premium for companies with an aggregated turnover of below AUD$20 million.
32
The refundable R&D tax offset is accounted for under IAS 20 Accounting for Government Grants and Disclosure of Government Assistance, as per which the R&D tax offset income is recognized when there is reasonable assurance that it will be received. It is recognized in the statement of comprehensive income in the same period that the related costs are recognized as expenses and relates to refundable amounts on approved expenses.
Borrowings (current and non-current)
Total Borrowings at December 31, 2024, were AUD$3,896,328 of which AUD$3,882,778 is current and AUD$13,550 is non-current, compared to AUD$3,843,599 as at June 30 2024, of which AUD$2,084,152 was current and AUD$1,759,447 was non-current. The increase in total borrowings is due to the issuance of convertible notes totaling AUD$747,262, accrued convertible note interest of AUD$97,266 and a net increase of accrued shareholder loan interest of AUD$298,726, less debt discount on convertible notes of AUD$1,090,893. In addition, all borrowings, other than 2 director loans, have been reclassified from non-current to current during the period June 30, 2024 to December 31, 2024, as their repayment date is less than 12 months after December 31, 2024.
Convertible notes and derivative liabilities
Convertible notes payable are financial instruments which contain a separate financial liability and equity instrument. Such financial instruments are accounted for separately dependent on the nature of their components. The identification of such components embedded within a convertible notes payable requires significant judgement given that it is based on the interpretation of the substance of the contractual arrangement. The convertible notes issued by the Company are considered to contain embedded derivatives. The embedded derivatives were measured at fair value upon initial recognition based on a Black-Scholes valuation model and separated from the debt component of the notes. The debt component of the notes is measured at residual value upon initial recognition. Subsequent to initial recognition, the embedded derivative components are re-measured at fair value at each reporting date while the debt components are accreted to the face value of the note using the effective interest rate through periodic charges to finance expense over the term of the note.
In accordance with IFRS 9, where an indeterminate number of shares may be issued in due course upon the conversion of the convertible notes, or the convertible notes are convertible at a discount to market, the embedded derivative is accounted for as a liability.
The Company’s shares attained a trading stock price upon the completion of the IPO and listing of the Company’s shares. As such, the Company is required to value and separately account for the derivative embedded within convertible notes issued by the Company. Please see Note 3 and Note 20 in the financial statement for the six months ending December 31, 2024 and 2023 commencing on page F-8 and page F-29 respectively.
Corporate and administrative expenses (in AUD)
|
Period ended |
||||
|
2024 |
2023 |
|||
|
AUD$ |
AUD$ |
|||
|
Employment expenses |
248,655 |
519,687 |
||
|
Corporate expenses |
456,743 |
98,419 |
||
|
IPO related expenses |
637,594 |
102,941 |
||
|
Depreciation and amortization expenses |
606,497 |
609,274 |
||
|
Advertising & marketing expense |
118,211 |
18,200 |
||
|
Consulting fees |
409,134 |
750 |
||
|
Other expenses |
203,680 |
25,527 |
||
|
Finance costs |
620,785 |
286,791 |
||
|
Total Corporate and administrative expenses |
3,301,299 |
1,661,589 |
||
During the period ended December 31, 2024, total corporate and administrative expenses increased by AUD$1,639,710 to AUD$3,301,299 relative to AUD$1,661,589 in the similar period last year.
The AUD$1,639,710 increase in corporate and administrative expenses during the period ended December 31, 2024, relative to December 31, 2023, was predominantly due to an increase in (i) IPO related expenses by AUD$534,653, (ii) consulting fees by AUD$408,384 due to an increase in external consultants used, (iii) corporate expenses by AUD$358,324, due to higher professional and management fees, (iv) finance costs by AUD$333,994 due to increase
33
in borrowing and (v) other expenses by AUD$178,153 during the period ended December 31, 2024 as compared to the period ended December 31, 2023. The increase in corporate and administrative expenses as compared to the period ended December 31, 2023 was offset by a decrease in employment expenses of AUD$271,032, due to fewer employees and consequently lower wages and salaries relative to the period ended December 31, 2023.
Liquidity and Capital Resources (in AUD$)
The following table summarizes our changes in working capital from June 30, 2024 to December 31, 2024:
|
December 31, |
June 30, |
Change |
||||||||||||
|
Current Assets |
AUD$ |
4,939,130 |
|
AUD$ |
303,227 |
|
AUD$ |
4,635,903 |
||||||
|
Current Liabilities |
AUD$ |
6,278,655 |
|
AUD$ |
3,866,065 |
|
AUD$ |
2,412,590 |
||||||
|
Working Capital |
AUD$ |
(1,339,525 |
) |
AUD$ |
(3,562,838 |
) |
AUD$ |
2,223,313 |
||||||
As at December 31, 2024, there is a deficit of current assets over current liabilities of AUD$1,339,525 (June 30, 2024: deficit of current assets over current liabilities of AUD$3,562,838), which resulted in an increase of AUD$2,223,313 in working capital as a result of a capital raise during the six-months ended December 31, 2024.
The following table summarizes our changes in working capital from June 30, 2024 to June 30, 2023:
|
June 30, |
June 30, |
Change |
|||||||||||||
|
Current Assets |
AUD$ |
303,227 |
|
AUD$ |
990,974 |
|
AUD$ |
(687,747 |
) |
||||||
|
Current Liabilities |
AUD$ |
3,866,065 |
|
AUD$ |
1,364,525 |
|
AUD$ |
2,501,540 |
|
||||||
|
Working Capital |
AUD$ |
(3,562,838 |
) |
AUD$ |
(373,551 |
) |
AUD$ |
(3,189,287 |
) |
||||||
As at June 30, 2024, there is a deficit of current assets over current liabilities of AUD$3,562,838 (June 30, 2023: deficit of current assets over current liabilities of AUD$373,551), however, we believe, that we would be able to meet our short-term obligations as they come due. The increase in the current liabilities for the year ended June 30, 2024 is due to outstanding shareholder loans with a balance of $1,923,000 due on December 31, 2024 which were reclassified as current liabilities from non-classified liabilities as at June 30,2024. The increase in the current liabilities should be viewed in light of the extension of due dates for the shareholder loans to December 31, 2025. In addition, on October 30, 2024, we consummated our initial public offering of 1,300,000 ordinary shares at a price of US$4.00 per share, generating gross proceeds to the Company of $5.2 million (approximately A$7.95 million) before deducting underwriting discounts and offering expenses.
The following table sets out information as to consolidated cash flow information for the years ended June 30, 2024 and 2023 in AUD$.
|
Years ended |
||||||||||
|
2024 |
2023 |
|||||||||
|
AUD$ |
AUD$ |
|||||||||
|
Net cash (used in) operating activities |
AUD$ |
(1,070,471 |
) |
AUD$ |
(1,770,435 |
) |
||||
|
Net cash (used in) investing activities |
AUD$ |
(148,170 |
) |
AUD$ |
(75,931 |
) |
||||
|
Net cash from financing activities |
AUD$ |
843,939 |
|
AUD$ |
2,030,547 |
|
||||
|
Net cash inflow/(outflow) |
AUD$ |
(374,702 |
) |
AUD$ |
184,181 |
|
||||
|
Effects of exchange rate changes on cash and cash equivalents |
AUD$ |
— |
|
AUD$ |
52,558 |
|
||||
|
Net increase/(decrease) in cash and cash equivalents |
AUD$ |
(374,702 |
) |
AUD$ |
236,739 |
|
||||
Years ended June 30, 2024 and 2023
As of June 30, 2024, we had cash and cash equivalents of AUD$24,522 compared to cash and cash equivalents of AUD$399,224 as of June 30, 2023. The decrease in cash and cash equivalents of AUD$374,702 is attributed to the following activities:
For the year ended June 30, 2024, net cash used in operating activities was AUD$1,070,471 relative to AUD$1,770,435 for the corresponding period last year, a decrease of AUD$699,964. The decrease in cash used in operating activity is primarily attributable to a decrease in payments to suppliers and employees of AUD$505,278 (June 30, 2024, AUD$1,372,801 compared to AUD$1,878,079 at June 30, 2023) and payment to initial public offering vendors of AUD$160,489 (June 30, 2024, Nil compared to AUD$160,489 at June 30, 2023).
34
For the year ended June 30, 2024, net cash used in investing activities increased by AUD$72,239 due to acquisition of intangible assets and property, plant and equipment.
For the year ended June 30, 2024, net cash from financing activities decreased by AUD$1,186,608 to AUD$843,939 (June 30, 2023: AUD$2,030,547) primarily due to the nil proceeds from share issuance (June 30, 2023: AUD$1,431,162) offset by capital issue cost of nil (June 30, 2023: AUD$121,844)
For the year ended June 30, 2024, effects of exchange rate changes on cash and cash equivalents decreased by AUD$52,558 to AUD$ nil (June 30, 2023: AUD$52,558) due to decrease in foreign currency transactions.
The following table sets out information as to consolidated cash flow information for the six months ended December 31, 2024 and 2023 in AUD$.
|
Period ended |
||||||||||
|
2024 |
2023 |
|||||||||
|
AUD$ |
AUD$ |
|||||||||
|
Net cash (used in) operating activities |
AUD$ |
(3,441,439 |
) |
AUD$ |
(429,172 |
) |
||||
|
Net cash (used in) investing activities |
AUD$ |
(328,222 |
) |
AUD$ |
(79,961 |
) |
||||
|
Net cash from financing activities |
AUD$ |
6,645,106 |
|
AUD$ |
236,693 |
|
||||
|
Net cash inflow/(outflow) |
AUD$ |
2,875,445 |
|
AUD$ |
(272,440 |
) |
||||
|
Effects of exchange rate changes on cash and cash equivalents |
AUD$ |
146,635 |
|
AUD$ |
— |
|
||||
|
Net increase/(decrease) in cash and cash equivalents |
AUD$ |
3,022,080 |
|
AUD$ |
(272,440 |
) |
||||
Six months ended December 31, 2024 and 2023
As at December 31, 2024, we had cash and cash equivalents of AUD$3,046,602 compared to AUD$126,784 as at December 31, 2023, an increase in cash and cash equivalents of AUD$2,919,818.
The increase in cash and cash equivalents is predominantly due to the following activities:
For the period ended December 31, 2024, net cash used in operating activities was AUD$3,441,439 relative to AUD$429,172 for the corresponding period last year, registering an increase of AUD$3,012,267. The increase in cash used in operating activity is predominantly due to an increase in payments to employees and suppliers of AUD$3,423,048 (December 31, 2023: AUD$732,093) offset by decrease in cash receipts from customers of AUD$Nil (December 31, 2023: AUD$40,000) and decrease in Research and Development tax incentives received of AUD$Nil (December 31, 2023: AUD$263,057).
For the period ended December 31, 2024, net cash used in investing activities was AUD$328,222 (compared to AUD$79,961 for the corresponding period ended December 31, 2023) due to an increase in acquisition of property, plant and equipment and intangibles.
For the period ended December 31, 2024, net cash from financing activities was AUD$6,798,322 (December 31, 2023: AUD$236,693) an increase of AUD$6,561,639. The difference is primarily driven by proceeds from issue of shares of AUD$7,913,463 at December 31, 2024, compared to AUD$Nil in the corresponding period last year and borrowing proceeds of AUD$747,261 as at December 31, 2024 compared to AUD$248,588 in the corresponding period last year, resulting in an increase of AUD$498,673, offset by proceeds of capital issue cost of AUD$1,862,392 (December 31, 2023: AUD$Nil)
For the period ended December 31, 2024, the difference in the effects of exchange rate changes on cash and cash equivalents was due to movements in USD/AUD exchange rates and amounts held in foreign currencies.
Cash Flow
In January 2023, we negotiated with holders of our unsecured loans to extend the terms of the loans for another 12 months on the same terms from July 2023 until July 2024. In October 2023, all holders of the unsecured loans have agreed to further extend the terms of the loans until December 31, 2024, which were further extended until December 31, 2025 by the agreement of all holders in October 2024. These extensions further helped reduce our
35
immediate or short term liabilities in the fiscal years ending June 30, 2023 and 2024. We expect to require further extensions for such loans for the year ending June 30, 2025 to reduce the short term liability if the Company determines this is needed.
On October 3, 2023, the Company closed the Convertible Note offering raising approximately AUD$1,004,889 (AUD$410,000 plus USD$400,000 calculated at the daily exchange rate when each amount was received). Each Convertible Note shall have a face value of AUD$1, an annual interest rate of 12% and have a maturity date of December 31, 2025. Each holder of a Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms of the Convertible Note, either elect to convert their Convertible Note into Ordinary Shares or redeem their Convertible Note for an Australian cash payment. The December 31, 2025 repayment date of the Convertible Notes were intended to alleviate the Company’s short term liabilities.
On March 26, 2024, the Company closed the February 2024 Convertible Note offering, raising AUD$357,338 (approximately AUD$75,000 plus approximately USD$185,000 calculated at the daily exchange rate when each amount was received). Each February 2024 Convertible Note shall have a face value of AUD$1, an annual interest rate of 6% and have a maturity date of December 31, 2025. Each holder of a February 2024 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms of the February 2024 Convertible Note, either elect to convert their February 2024 Convertible Note into Ordinary Shares or redeem their February 2024 Convertible Note for an Australian cash payment. The December 31, 2025 repayment date of the February 2024 Convertible Notes are intended to alleviate the Company’s short term liabilities.
On May 27, 2024, the Company’s board of directors approved the issuance of convertible notes (the “May 2024 Convertible Note”) to raise up to AUD$1,000,000. Each May 2024 Convertible Note had a face value of AUD$1, an annual interest rate of 6% and have a maturity date of December 31, 2025. Each holder of a May 2024 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms therein, either elect to convert their May 2024 Convertible Note into Ordinary Shares at a conversion discount rate of 22% or redeem their May 2024 Convertible Note for an Australian cash payment. The Company has received approximately AUD$1million (approximately AUD$315,000 plus approximately USD$450,000 calculated at the daily exchange rate when each amount was received) through the issuance of the May 2024 Convertible Notes.
The Company closed its initial public offering on October 30, 2024, issuing 1.3 million ordinary shares at an issue price of US$4.00 per share and raising USD$5.2 million (approximately AUD$8.00 million) before deducting underwriting discounts and offering expenses.
To reduce the Company’s debt position and improve its balance sheet, the Company in January 2025 offered existing convertible note and shareholder loan holders the ability to convert their loans into equity, be repaid or continue to maturity. For the then outstanding convertible notes, a total of AUD $822,184 (approximately USD$534,420) was converted in March 2025 by the election of the noteholders into Ordinary Shares at a share price of USD$2.14. In March 2025, the Company paid to loan holders an aggregate of AUD$772,136 (approximately USD$501,888) in order to redeem their loans. The remaining principal and interest on the outstanding shareholder loans will accrue until maturity in December 2025.
On February 21, 2025, our board of directors approved the issuance of convertible notes (the “February 2025 Convertible Note”) to raise up to AUD$1,500,000. Each February 2025 Convertible Note had a face value of AUD$1, an annual interest rate of 20% and have a maturity date of July 1, 2026. Each holder of a February 2025 Convertible Note may at any time elect to convert their February 2025 Convertible Note into Ordinary Shares at a conversion price of USD$2.00. Each holder of a February 2025 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms therein, either elect to convert their February 2025 Convertible Note into Ordinary Shares at a conversion price of USD$2.00 or redeem their February 2025 Convertible Note for an Australian cash payment. As of the date of this prospectus, the Company has received approximately AUD$580,000 (approximately USD$377,000) through the issuance of the February 2025 Convertible Notes.
We do not expect to require additional capital apart from the proceeds of this offering should our operations continue as forecasted. Should we experience lower than expected sales volumes or lower growth opportunities, then we may be required to consider additional financing options to continue the Company’s growth to achieve positive cash flow. However, we intend to adjust our expenses to align with the revenue generated to ensure we remain financially solvent. See further discussion within the section “Risk Factors — There is substantial doubt about our ability to continue as a going concern.”
36
To the extent that we raise additional capital through the sale of equity or convertible debt securities, the issuance of such securities could result in substantial dilution for our current shareholders. The terms of any securities issued by us in future capital transactions may be more favorable to new investors, and may include preferences, superior voting rights and the issuance of warrants or other derivative securities, which may have a further dilutive effect on the holders of any of our securities then-outstanding. We may issue additional Ordinary Shares or securities convertible into or exchangeable or exercisable for our Ordinary Shares in connection with hiring or retaining personnel, option or warrant exercises, future acquisitions or future placements of our securities for capital-raising or other business purposes. The issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our Ordinary Shares to decline and existing shareholders may not agree with our financing plans or the terms of such financings. In addition, we may incur substantial costs in pursuing future capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs. We may also be required to recognize non-cash expenses in connection with certain securities we issue, such as convertible notes and warrants, which may adversely impact our financial condition. Furthermore, any additional debt or equity financing that we may need may not be available on terms favorable to us, or at all. If we are unable to obtain such additional financing on a timely basis, we may have to curtail our research and development activities and growth plans and/or be forced to sell assets, perhaps on unfavorable terms, or we may have to cease our operations, which would have a material adverse effect on our business, results of operations and financial condition.
Qualitative and Quantitative Information on Financial Risks
Financial Risk Management, including market risk (foreign currency risk, price risk and interest rate risk)
Our activities expose us to a variety of financial risks: market risk (including foreign currency risk, price risk and interest rate risk), credit risk and liquidity risk.
Our overall risk management program focuses on the unpredictability of financial markets and seeks to minimize potential adverse effects on the financial performance of the Company.
We use different methods to measure different types of risk to which it is exposed. These methods include sensitivity analysis in the case of interest rate, foreign exchange and other price risks, ageing analysis for credit risk and beta analysis in respect of investment portfolios to determine market risk.
Market risk
Foreign currency risk
We have only very minor exposure to foreign currency risk. Foreign exchange risk arises from future commercial transactions and recognized financial assets and financial liabilities denominated in a currency that is not the entity’s functional currency. The risk is measured using sensitivity analysis and cash flow forecasting. Management understands that, over the next twelve months, it will deal in a much greater volume in foreign currencies and are in the process of having in place a risk management policy accordingly.
Price risk
We are not exposed to any significant price risk.
Cash flow and fair value interest rate risk
We have limited exposure to interest rate risk arising from long-term borrowings as these are based on fixed rates. There are no borrowings obtained at variable rates for the six months ended December 31, 2024 and 2023 or in the year ended June 30, 2024 or June 30, 2023. All cash is held in checking accounts or on hand, and do not earn interest.
Credit risk
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the group. The maximum exposure to credit risk at the reporting date to recognized financial assets is the carrying amount, net of any provisions for impairment of those assets, as disclosed in the statement of financial position and notes to the financial statements. The Company does not hold any collateral.
37
All trade and other receivables are current as at December 31, 2024, June 30, 2024 and June 30, 2023, with no balances past due.
The Company recorded no bad debt expense in the six months ended December 31, 2024 and December 31, 2023 and the years ended June 30, 2024 or June 30, 2023. As of December 31, 2024 and 2023, and June 30, 2024 and 2023, there was no expected credit losses recorded.
Generally, trade receivables are written off when there is no reasonable expectation of recovery. Indicators of this include the failure of a debtor to engage in a repayment plan, no active enforcement activity and a failure to make contractual payments for a period greater than 1 year.
Liquidity risk
Vigilant liquidity risk management requires the Company to maintain sufficient liquid assets (mainly cash and cash equivalents), and available borrowing facilities to be able to pay debts as and when they become due and payable. The Company manages liquidity risk by maintaining adequate cash reserves and available borrowing facilities by continuously monitoring actual and forecast cash flows and matching the maturity profiles of financial assets and liabilities.
Borrowings as at December 31, 2024, June 30, 2024 and June 30, 2023are fully drawn.
Remaining contractual maturities
During the year ended June 30, 2022, the Company entered into a lease agreement with Lifestyle Breakthrough Holdings U/T (“Lifestyle”) to rent office space. Lifestyle is an entity associated with Nathan J. Givoni and Simon H. Szewach, both are which are directors of the Company.
The Company entered into a lease agreement for office space for a term of 24 months with the first three months of the lease provided as a rent-free period. The lease has expired on November 1, 2023 and the Company is continuing the former lease on a month to month basis. The Company expects to terminate such lease agreement at the end of the financial year ending June 30, 2025. The total rental payable was nil as at June 30, 2024 and $11,896 at June 30, 2023.
The following tables detail the Company’s remaining contractual maturity for its financial instrument liabilities for the year ended June 30, 2024 and 2023. They have been drafted based upon the undiscounted cash flows of financial liabilities based on the earliest date on which the financial liabilities are required to be paid. The tables include both interest and principal cash flows disclosed as remaining contractual maturities. Therefore, these sums may differ from their carrying amount in the statement of financial position for the same corresponding years ended.
|
Consolidated – June 30, 2023 |
Weighted |
1 year or |
Between 1 |
Between 2 |
Over 5 years |
Remaining |
|||||||
|
% |
AUD$ |
AUD$ |
AUD$ |
AUD$ |
AUD$ |
||||||||
|
Non-derivatives |
|
||||||||||||
|
Non-interest bearing |
|
||||||||||||
|
Trade and GST payables |
— |
|
314,425 |
— |
— |
— |
314,425 |
||||||
|
Payroll liabilities |
— |
|
292,755 |
— |
— |
— |
292,755 |
||||||
|
Other loans |
— |
|
5,086 |
13,550 |
— |
— |
18,636 |
||||||
|
|
|||||||||||||
|
Interest-bearing – fixed rate |
|
||||||||||||
|
Lease liability |
4.2 |
% |
12,000 |
— |
— |
— |
12,000 |
||||||
|
Borrowings |
0.50 |
% |
— |
155,304 |
— |
— |
155,304 |
||||||
|
Borrowings – loans |
12.00 |
% |
— |
1,938,287 |
— |
— |
1,938,287 |
||||||
|
Borrowings – Convertible notes |
12.00 |
% |
— |
— |
1,095,452 |
— |
1,095,452 |
||||||
|
Total non-derivatives |
|
624,266 |
2,107,141 |
1,095,452 |
— |
3,826,859 |
|||||||
38
|
Consolidated – June 30, 2024 |
Weighted |
1 year or |
Between 1 |
Between 2 |
Over 5 years |
Remaining |
|||||||
|
% |
AUD$ |
AUD$ |
AUD$ |
AUD$ |
AUD$ |
||||||||
|
Non-derivatives |
|
||||||||||||
|
Non-interest bearing |
|
||||||||||||
|
Trade and GST payables |
— |
|
387,034 |
— |
— |
— |
387,034 |
||||||
|
Payroll liabilities |
— |
|
702,619 |
— |
— |
— |
702,619 |
||||||
|
Other loans |
— |
|
5,086 |
13,550 |
— |
— |
18,636 |
||||||
|
|
|||||||||||||
|
Interest-bearing – fixed rate |
|
||||||||||||
|
Borrowings |
0.50 |
% |
156,066 |
— |
— |
— |
156,066 |
||||||
|
Borrowings – loans |
12.00 |
% |
1,938,778 |
— |
— |
— |
1,938,778 |
||||||
|
Borrowings – Convertible notes |
12.00 |
% |
— |
1,307,573 |
— |
— |
1,307,573 |
||||||
|
Borrowings – Convertible notes |
6.00 |
% |
— |
670,743 |
— |
— |
670,743 |
||||||
|
Total non-derivatives |
|
3,189,583 |
1,991,866 |
— |
— |
5,181,449 |
|||||||
Critical accounting estimates and judgements
The preparation of the consolidated financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results. The judgements, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities within the next financial year are summarized below.
Impacts of Coronavirus (COVID-19)
Judgement has been exercised in considering the impacts that the COVID pandemic has had, or may have, on the Company based on known information. This consideration extends to the nature of the products and services offered, customers, supply chain, staffing and geographic regions in which the Company operates. Other than as addressed in specific notes, there does not currently appear to be either any significant impact upon the financial statements or any significant uncertainties with respect to events or conditions which may impact the Company unfavorably as at the reporting date or subsequently as a result of the COVID-19 pandemic.
Estimation of useful lives of assets
The Company determines the estimated useful lives and related depreciation and amortization charges for its finite life intangible assets. The useful lives of such assets could change significantly due to events such as technical innovations. The depreciation and amortization charge will increase where the useful life of an asset is less than previously estimated, or technically obsolete or non-strategic assets that have been abandoned or sold will be written off or written down.
Intangible assets
The Company tests annually, or more frequently if events or changes in circumstances indicate impairment, whether indefinite life or finite life intangible assets have suffered any impairment, in accordance with the accounting policy stated in Note 3 in the December 31, 2024 and June 30, 2024 and June 30, 2023 financial statements. The recoverable amounts of cash-generating units have been determined based on a fair value less cost to sell calculations. These calculations require the use of assumptions, including estimated discount rates based on the current cost of capital and growth rates of the estimated future cash flows.
39
Impairment analysis conducted at the period ended December 31, 2024
For the period ended December 31, 2024, we had prioritized pharmaceutical research and improving operational processes, and we expect to grow and execute on our business plans with lower overheads and expenses in the financial year ending June 30, 2025. To facilitate this, we entered into a rental contract for laboratory facilities with Monash University on February 2, 2024 (the “Monash Facilities”) for further research purposes. Our lack of personnel, our focus on research, and identifying and establishing a laboratory facility, adversely affected our ability to entered new sales opportunities which resulted in nil sales revenue for the period ended December 31, 2024 and 2023.
With the Monash Facilities established and fitted, we are resuming our sales activities with a focus on the nutraceutical and sports vertical for the year ending June 30, 2025. For the year ended June 30, 2026, we expect to add animal health, over-the-counter and pharmaceuticals verticals to our sales activities. Notwithstanding the foregoing, we are currently prioritizing pharmaceutical research on our existing 505b(2) application and seeking other potential pharmaceutical candidates through such pathway.
The delay in completing the January 2023 orders, and recognizing the relevant component of the outstanding deferred revenue balance as income, is due to certain of our clients which experienced cash flow difficulties and was not able to pay for their outstanding orders. However, such orders are now manufactured and shipped in the fiscal year ending June 30, 2025. We have also put in place more rigorous qualification procedures to ensure customers have the financial ability to proceed with orders through to manufacturing in a timely manner. In the event that cash flow difficulties continue for such customers, we have instituted a policy where all orders and deposits are non-refundable and we will not process refunds for non-payments. As a result of the foregoing delays, we have not recognized revenues from undelivered orders for the period ended December 31, 2024 and we expect to recognize such revenues upon delivering these orders for the year ending June 30, 2025.
The delayed processing of orders does not adversely affect our assumptions underpinning the valuation model and analysis as used at December 31, 2024. The recoverable amount of the cash generating unit has been determined by a forecast model that estimated the future cash flows based on budgets and forecasts for five years prepared by our management. As part of a valuation of the intangible assets performed by an independent expert valuer as at June 30, 2024, such experts extended the forecasts for an additional four years for a total forecast period of nine years on the basis that, for early stage businesses that are reasonably expected of high growth for a significant period of time, it is generally necessary to forecast cash flows for a period greater than five years to reflect the business reaching a mature stable level of growth to apply a terminal value calculation. For the purposes of the assessment of the coverable amount of the cash generating unit as at December 31, 2024, our management has continued to adopt this approach, on the basis that, as at that date, we remain an early stage business with the same characteristics it had when the expert valuer performed the previous valuation.
Further, included at the end of the forecast period is a terminal value reflecting a continuing value at the end of the forecast period on the basis of capitalizing free cash flows in perpetuity at a growth rate of 2.5% per annum. These cash flows were then discounted to their present value using a discount rate that reflects current market assessments of the time value of money and the risks specific to the cash generating unit. The sensitivity analysis performed continued to show significant headroom over the carrying value of the intangible assets at December 31, 2024, notwithstanding revenue was nil for the period ended December 31, 2024.
In terms of product and sales development, and in support of the foregoing assumption in revenue opportunities, for our analysis for the period ended December 31, 2024, we have identified potential customers for the financial year ending June 30, 2026. We have discussed with such potential customers on product opportunities, product type, potential quantities and the timing of orders. We believe we have the potential to generate revenue from such customers, through customers that have placed or are about to place a purchase order with us.
As we continue to grow and invest in sales and marketing, we expect to identify additional customers. We intend to use the net proceeds from our initial public offering and this offering to generate revenue by investing in sales, marketing and manufacturing to leverage commercial opportunities to generate the expected increase in future revenue growth as compared to the period ended December 31, 2024.
For the period ended December 31, 2024, we generated no revenue. We are an early-stage company and it is reasonably expected that we will have significant revenue growth during our early years. As such, we have shipped over 400,000 units for the fiscal year ending June 30, 2025 from prior orders.
40
The potential customers which we have identified as revenue opportunities for the financial years ended June 30, 2026 and 2027 would expect to underpin the revenue growth in the next two years. Our forecasts of annual revenue growth is 259% for the financial year ended June 30, 2026, approximately 117% for the financial year ended June 30, 2027, 86% for the financial year ended June 30, 2028 and 38% for the financial year ended June 30, 2029.
For the financial year beginning July 1, 2027, the Company’s incremental annual revenue growth is supported by an analysis of additional revenue opportunities through scaling up and expected increased market penetration in the existing nutraceutical, pharmaceutical and animal nutraceutical markets.
Impairment analysis conducted at the year ended June 30, 2024 and June 30, 2023
We performed an impairment assessment based on the cash generating unit (“CGU”) as at June 30, 2024 and as at June 30, 2023. We determined that the recoverable amount in relation to the CGU exceeded its carrying value of assets as at June 30, 2024, and also as at June 30, 2023. Therefore, there was no requirement to adjust the carrying value for either financial year.
Our directors reviewed and agreed with the significant assumptions determined by our management. As such, our directors believed that no impairment charge was required to the value of the intangible asset at June 30, 2024 or as at June 30, 2023.
Please refer to Note 20 in the financial statements for the years ended 30 June 2024 and 2023 (per page (F-69) of this prospectus) for further information about the assumptions used in such assessments.
Recognition of deferred tax assets
Deferred tax assets are recognized for deductible temporary differences and carried forward losses, only if the Company considers it is probable that future taxable amounts will be available to utilize those temporary differences and losses.
Leases — Incremental borrowing rate
Where the interest rate implicit in a lease cannot be readily determined, an incremental borrowing rate is estimated to discount future lease payments to measure the present value of the lease liability at the lease commencement date. Such a rate is based on what the Company estimates it would have to pay a third party to borrow the funds necessary to obtain an asset of a similar value to the right-of-use asset, with similar terms, security and economic environment.
Employee benefits provision
As discussed in Note 3 of the December 31, 2024, June 30, 2024 and June 30, 2023 financial statements the liability for employee benefits expected to be settled more than 12 months from the reporting date are recognized and measured at the present value of the estimated future cash flows to be made in respect of all employees at the reporting date. In determining the present value of the liability, the Company has considered the estimates of attrition rates and pay increases through promotion and inflation.
Going Concern — for the six months ended December 31, 2024
The working capital position as at December 31, 2024 of the Company results in an excess of current liabilities over current assets of $1,339,525 (June 30, 2024: excess of current liabilities over current assets of $3,562,838). The Company had a loss, after income tax, of $3,304,359 during the six-month ended December 31, 2024 (six-month period ended December 31, 2023 loss: $1,685,644). As of December 31, 2024, there were no capital commitments outstanding. The cash balances as at December 31, 2024 was $3,046,602 (June 30, 2024: $24,522).
The above matters give rise to a material uncertainty that may cast significant doubt over the Company’s ability to continue as a going concern. Therefore, the Company may be unable to realize its assets and discharge its liabilities in the normal course of business at the amounts stated in the consolidated financial statements.
41
Notwithstanding the foregoing, the board of directors believe that it is reasonably foreseeable that the Company will be able to continue as a going concern and that it is appropriate to adopt the going concern basis in the preparation of the financial statements, after considering the following matters:
• The board of directors have prepared detailed cash flow projections for a period of at least 12 months from the date of signing the consolidated financial statements for the six months ended December 31, 2024.
• The Company’s ability to fund its operations is dependent upon management’s plans and execution, which include raising additional capital, if required, through public and other offerings, obtaining regulatory approvals for its products and generating revenues from these products and having the ability to be able to reduce expenditures accordingly if required, in order to be able to pay its debts as and when they fall due.
• On February 21, 2025 the Company’s board of directors approved by resolution to raise up to AUD$1,500,000 in convertible notes with a maturity date of July 1, 2026 such that the Company may continue to operate as a going concern.
• On March 13, 2025, the Company signed the Purchase Agreement, whereby the Company may receive gross proceeds of up to USD$12,000,000 from the sale of Ordinary Shares to Lincoln Park under the Purchase Agreement, from time to time, at our discretion after a registration statement is declared effective and after satisfaction of other conditions in the Purchase Agreement.
The Company’s six month condensed consolidated financial statements for December 31, 2024 have been prepared on a going concern basis which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. The six month condensed consolidated financial statements for December 31. 2024 do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities should the Company be unable to continue as a going concern.
Discussion of Going Concern for the financial years ended June 30, 2024 and June 30, 2023
There are circumstances that give rise to a material uncertainty that may cast significant doubt over the Company’s ability to continue as a going concern for both the financial years ended June 30, 2024 and June 30, 2023.
Refer to note 4 in the financial statements for the years ended June 30, 2024 and 2023 (per page (F-62) of this prospectus) for further information in regard to these circumstances.
42
BUSINESS
Business Overview
We are a clinical and science-based company that is focused on developing and commercializing white label gel-based delivery solutions for prescription drugs, nutraceuticals, pet care and other products. A “white label” gel-based delivery solution is where we produce a product that other companies rebrand as their own product. Our principal products are edible gels, which we refer to as gels, and their application in gel-based dosage forms. Our current product suite consists of multiple products that sit within five core verticals — for pets, sports, pharmaceutical (pharma), over-the-counter (OTC) and nutraceutical — all of which leverage our patent pending multiple-ingredient dosage forms, and that we expect to have a wide range of applications and consumers. We currently focus our efforts on out-licensing our technology to companies to develop and create new products they can manufacture and sell within their established and researched markets, while we continue to manufacture our existing products under license (“white label”). Our vision is to change the way good health is delivered to both humans and animals through our patent pending multiple-health-ingredient gel dosage forms.
Of our products already licensed, three clients have placed initial orders for nutraceutical products, and there have been four other products in the sports vertical ordered. Such orders were placed in fiscal year ended June 30, 2024. With regards to the pets, nutraceutical and sports vertical, we designed these products to have no regulatory hurdles to overcome as they have food grade classifications and therefore do not require regulatory approvals. We designed our gel platform to enhance the tolerability and stability of drugs while maintaining their efficacy. Products in the pharma vertical will require regulatory approval.
For the fiscal year ended June 30, 2025, we have prioritized research and development work, our initial public offering and the required adjustments to our team and operations to allow us to effectively achieve sales target of our products. Such efforts included wider sales and marketing opportunity in the Asia Pacific region with WPIC to facilitate initial sales of the sports vertical products there.
We have prepared and applied for patents which relate to a diagnostic gel product comprising glucose, and certain multiple health ingredient dosage forms. Our first patent family is comprised of granted U.S. patent 10,983,132, the People’s Republic of China patent CN108289963B, Australia patent 2016351301, European Patent Office patent 3370776 and India patent 514796 which is for an oral glucose tolerance test gel and testing method for diabetes diagnostics. As of the date of this prospectus, we have applications in Canada and Qatar. We intend to protect products that employ our gel technology in our second patent family which is directed to certain multiple-health ingredient dosage forms which utilize a gel formulation that features agarose and alginate that in certain ratios and pH ranges form gels of specific firmness to deliver two or more health ingredients (including medicines) in a single dosage form. This second patent family is comprised of the granted European Patent Office patent 3809877, Mexico patent 416876, Israel patent 278541, Hong Kong patent 40051090, United Kingdom patent 3809877 and patent pending applications in the following countries: Australia, Brazil, Canada, the Eurasian Patent Organization, India, Japan, South Korea, the People’s Republic of China, and the United Arab Emirates, the United States, and South Africa.
We continue to work on preparing additional patent applications. Our third patent application addresses challenges with delivering oil-based products in gels, our fourth patent application covers products produced for the nutritional health dysphagia market where swallowing tablets is challenging, and our fifth patent application addresses pharmaceutical formulations with the delivery of a single Active Pharmaceutical Ingredient (API). These applications have been lodged as provisional patents in the United Kingdom in August 2022, December 2022 and May 2023, respectively. We have also lodged as provisional patents in the United Kingdom in December 2024 our sixth and seventh patent applications which addresses pharmaceutical formulations with the delivery of an Active Pharmaceutical Ingredient (API) and an eighth patent application which addresses various textures for the delivery of an Active Pharmaceutical Ingredient (API).
Our History
Gelteq as an entity began in October 2018, but the initial research work commenced in 2014 by Gelteq co-founder Mr. Nathan J. Givoni
In January 2015, Mr. Givoni began his long-term collaboration with Monash University in Melbourne, Australia, to verify and test our gel formulations. Our company’s first patent family relates to an oral glucose tolerance test gel and testing method for diabetes diagnostics and commenced as a provisional patent in Australia in 2015, which continued to
43
be evaluated and tested before it was submitted as a standard patent application in Australia in 2016. For this first patent family, U.S. patent 10,983,132, the People’s Republic of China patent CN108289963B and Australia patent 2016351301 have been granted with several patent applications pending in a number of foreign countries. This glucose tolerance test gel was the subject of a pilot project, after which the focus shifted to establishing strategic partnerships to further develop industry-specific products, which were nutraceutical formulations such as sugar lowering products for people with pre-diabetes. The creation of these products did not require specific regulatory approvals. In 2018, Mr. Simon H. Szewach joined the business and our second patent family was later lodged provisionally in Australia, with a further standard patent application submitted in 2019 in the U.S. and a number of foreign countries. The patent applications of our second patent family are granted by the European Patent Office 3809877 with several patent applications pending in a number of foreign countries. The patent applications are directed to certain multiple-health ingredient gel dosage forms to utilize our gel delivery technology. By 2020, these two patent families had been acquired by Gelteq after it was co-founded by Mr. Givoni and Mr. Szewach. The primary focus of Gelteq has been delivering and creating new and innovative products that utilize our gel-based technologies. Utilizing the acquired intellectual property, Gelteq completed product research in early 2020 for a suite of nutraceutical products and since that time, has introduced its first product line and actively pursued (through further research and development), additional applications for the gel technology, which is specifically suited for sports, pharmaceutical (pharma) and over-the-counter (OTC) usage.
Our Strengths
We are seeking to position ourselves as a leader in the application of ingestible gel technology in nutraceutical, drug and supplement delivery in the following manner:
• seeking to position ourselves as an emerging market leader in dosage forms that utilize ingestible gel technology for nutraceutical, pet care, and pharma;
• promoting our products as superior to other methods of oral delivery (i.e., pills, tablets, gummies);
• highlighting our products as addressing unmet issues around swallowing, taste, dosage and efficacy;
• taste-masking ability of Gelteq’s patent pending multiple-health ingredient gel dosage forms, being able to immediately address unsolved challenges in compliance and dosing;
• creating manufacturing and distribution and sale channels permits expedited time-to-market for high-demand products;
• expanding our intellectual property portfolio by maintaining our 100%-owned U.S. patent for a glucose tolerance testing product, and working to have our additional pending patent applications inside and outside of the United States proceed towards allowance, and filing additional patent applications to protect our new discoveries;
• maintaining our research partnership with Australia’s Monash University, which is ranked among the top universities in the world in pharmaceutical science by the 2023 QS World University Rankings for Pharmacy & Pharmacology and is providing more opportunities in the expanded field of animal husbandry, while negotiating another research partnership with another Australian university’s veterinary hospital; and
• signing industry partnerships/licenses for pilot programs with our licensee companies for sport-related gels described herein under “Business — Material Contracts — Customer Contracts.”
Our Strategy
Overall
The following are highlights of our strategy to promote and expand our business at the present time:
• Greatest unmet demand for our gel dosage forms — We will focus on dysphagia (the medical term given to difficulty swallowing) and other areas including children and seniors where the need is great and current solutions inadequate. See “Business — Human Market Insights — Gels directly combat the problems associated with Dysphagia” for a discussion of dysphagia.
44
• Fastest ability to grow sales — we are looking to capitalize on existing opportunities in the market.
• Highest margins — certain markets, such as pet nutrients, nutraceuticals and human supplements, offer high margins.
• Little to no competitors — We are seeking “blue ocean” markets where the competition is not currently focusing, including in the pharmaceutical (pharma) and over-the-counter (OTC) markets.
• Highest Demand for a market differentiating delivery platform — issues such as difficulty in swallowing, need to intake a large amount of drugs or nutrients, and taste making are all areas where our product can show deep differentiation and shine.
Based on this, we have decided to focus our efforts in the following order at the present time:
• First, — nutraceuticals — We have created formulations for products in the nutraceutical sector that include dietary fiber, prebiotics, probiotics, vitamins, polyunsaturated fatty acids, antioxidants, electrolytes and others. At this stage, our nutraceuticals had been created from our laboratories, flavored and shown to be shelf stable by our manufacturers and are ready to be sold to the public. We have also already sold products in our sports vertical which contain electrolytes and carbohydrates as primary ingredients to PacificPine Tennis Limited, PacificPine Football Limited, PacificPine Golf Limited and Five-Star Sports Hong Kong Limited. We have also sold a product that addresses brain function in our nutraceutical vertical, taking a proprietary powder blend owned by Healthy Extracts Inc. (OTCQB:HYEX) and creating an easy to consume gel product for Healthy Extracts Inc. and their customers. In addition, we have sold 20,000 units of Hypogel in our nutraceutical vertical, our product formulation that acts as a glucose boost to Lifestyle Breakthrough Holdings Pty Ltd. For these clients, we shipped an aggregate of 265,000 units for year ended June 30, 2022, with all these shipped products now recognized as revenue during the financial year ended June 30, 2022. The remainder orders continue to be held as deferred revenue, of which the Company has fulfilled 60,000 units in December 2022 and expects to fulfill the outstanding orders of 45,000 products in the fiscal year ending June 30, 2025. In January 2023, an existing customer placed further orders for two new products that respectively aids gut health and lowers sugar absorption, totaling 120,000 units, of which we received a AUD$45,437 (USD$29,534) non-refundable deposit for such orders in May 2023, and a new Australian client placed an order for 80,000 units for the product that lowers sugar absorption all in our nutraceutical vertical. For the year ended June 30, 2023, the 60,000 units delivered in December 2022 has been recognized as revenue of AUD$79,843 (USD$51,898) from the deferred revenue balance at June 30, 2022. We had experienced delays on delivering outstanding orders due to certain of our clients certain postponing their orders due to cash flow difficulties. We have shipped such orders in the fiscal year ending June 30, 2025 as our clients have resolved their cash flow issues. We have also put in place greater rigorous qualification procedures to ensure future customers have the financial ability to fund orders through manufacturing in a timely manner.
Nutraceuticals as a category also covers sports-related products. Our initial focus on nutraceuticals was due to larger market interest in the category and the recent engagement of WPIC Marketing and Technologies (“WPIC”) in December 2024 to assist with sales and distribution of its product throughout the Asia Pacific region. In February 2025, we have created the SportsGel brand, which will be the focus of WPIC’s sales in the Asia Pacific region initially. SportsGel was established to provide sports specific products all under one core brand to make it easier for third-party partners to sell. We currently have further product formulations in research and development for the financial year ending June 30, 2025, and are available as samples. We expect to enter new product formulations when we engage a potential license partner.
• Second, pet health/supplements — We have created products that comprise health ingredients related to joint health, coat quality, immune boosting, weight loss, diabetes and digestion for felines and canines. We have completed the research of the product formulations and they are awaiting future production at scale in their current form. Alternatively, their formulations can be adjusted by a future third-party license partner if so desired. As of the date of this prospectus, our pet health and supplemental products had been created from our laboratories, flavored and shown to be shelf stable by our laboratory tests and are ready to be produced at manufacturers before being sold to the public. We have tested samples of the canine and feline products respectively on canines and felines to verify acceptance and palatability. Further, we expect
____________
8 Ibid.
45
to begin formal studies for canine products in the fiscal year ending June 30, 2026. We took the decision to delay the pet health study to prioritize additional patent and formulation protections and to strengthen our IP portfolio which is intended to facilitate our expansion into the pharmaceutical sector.
• Third, healthcare/pharma — These could include pharmaceutical products for both human and pets, including those for people with swallowing issues. In our lab, we have created several pharmaceutical products for treatment of pain which have undergone dissolution studies. We expect one of these products we expect will soon be entered into the human 505(b)(2) pathway with the FDA, and potentially equivalent regulatory bodies in other regions. We also expect to work with license partners to create additional pharmaceutical products for human or animals which would require regulatory approval once developed. These future products potentially include gel dosage forms comprising a new API of a future licensing partner, which would require an NDA, or, for approved APIs, the 505(b)(2) pathway can be pursued. In the animal pharmaceutical space, we have received approval for our suitability petition for a new animal drug under development in December 2024. The new animal drug leverages our ingestible gel platform designed for nutrient and drug delivery. A suitability petition is a request by a drug sponsor to submit an abbreviated new animal drug application (“ANADA”) for a proposed innovative new animal drug that differs from a previously FDA approved generic animal drug. We have proposed changing the reference drug from a pill form into an oral gel form. These changes could be considered through the suitability petition, as defined under section 512(n)(3) of the Federal Food, Drug, and Cosmetic Act (the “FD&C Act”). The FDA concluded that the proposed changes did not require us to conduct further investigations to show the safety and effectiveness of the innovative new animal drug for its intended uses. Therefore, the FDA approved the petition under section 512(n)(3)(C) of the FD&C Act which foregoes the safety and effectiveness studies and helps reduce the timeframe to reach potential approval of the new animal drug. This approval of the suitability petition, however, does not guarantee approval of the ANADA for our proposed generic new animal drug.
Strategy Steps
Gelteq’s strategy is based on delivering innovative gel dosage forms that change the way good health is delivered to both humans and animals through our patent pending multiple-health-ingredient gel dosage forms. To achieve this objective, we intend to pursue the following:
• Maximize the commercial potential of our animal health and nutraceutical products through licensing and partnerships. We will continue to focus on white label and private label manufacturing using our patent pending multiple-health ingredient gel dosage forms, and then leveraging the brand awareness of the licensee and their existing customer base to ensure greater volumes of products are sold and then reordered from Gelteq. We began building relationships with animal health companies initially, closely followed by pharmaceutical companies, nutrition providers and sports organizations through which our products will be sold.
• Obtain FDA approval for our own gel-based drug dosage forms, through the 505(b)(2) pathway. To target the pain management market, we are currently taking an off-patent API for treatment of pain down the 505(b)(2) pathway and have completed dissolution studies. This has the potential, if approved by the FDA, to be available as our own gel-based OTC product with potential options to license-out or sell ourselves to consumers, or through a range of distributors. For this API candidate, we have completed dissolution comparisons to existing market products so that our future clinical data can be compared in bioequivalence studies to an existing, FDA approved product containing the same API. We have yet to perform further pre-clinical and clinical studies on bioequivalence and safety in humans which are required for FDA approval of different dosage forms. These clinical studies are expected to be run concurrently to further stability testing, with our initial lab stability data not indicating any lack of stability. Our API pipeline includes a further prescription medication API candidate that, once its dissolution study is completed, and its results are analyzed and collated, we expect to proceed with as described above for the OTC API.
• Expand our product suite to be made available to potential licensees. We will continuously conduct research and development and evaluate opportunities to leverage our gel delivery technology and patent pending multiple-health ingredient gel dosage forms, to develop additional products within pharmaceutical, nutraceutical, OTC and prescription markets.
46
• Complete clinical testing of our gel delivery technology with a variety of APIs. We are currently working on a multitude of pharmaceutical APIs that are available in different chemical structures, prioritizing dysphagia-based APIs, where we believe there is the greatest unmet need for an oral drug delivery system that has the potential to overcome the challenges of swallowability, taste, dosage and efficacy.
Our Products
All of Gelteq’s products currently are white label gel-based delivery solutions which third parties can use to create their own health products.
Gelteq patent pending multiple-health ingredient gels dosage forms are organized into three groups:
• Pet gels;
• Pharmaceutical/OTC gels; and
• Nutraceuticals/sports nutrition gels.
These multiple-health ingredient gel dosage forms are available for licensees to use “off the shelf.” However, if the licensee needs a special formulation, Gelteq will work with them to create a suitable gel product that meets their needs.
Gel Delivery System Details:
How It Works
The Gelteq Delivery System provides pharma and nutraceutical enhancements throughout every stage of ingestion in both animal and humans; addressing the complete experience — from the point of ingestion to final absorption:
• Mouth — Gelteq gels have the ability to moderate and mask poor-tasting, unsavory ingredients.
• Throat — Our “set” gel flows quickly, with a low internal resistance; inducing the swallowing reflex making it much more difficult to choke, especially compared to pills or capsules.
• Digestion — Gelteq gels easily breaks down within the digestive system; the gel protects nutrients or medicines from degradation and shields against stomach acids; ensures precise dosage is delivered.
• Gastrointestinal System — Gelteq gels can be modified to be fast or slow releasing, meaning quickly or slowly absorbed by adjusting the texture and a base set of ingredients of the gel system which can slow down the nutrient release; the gels target ideal absorption areas along digestive tract.
Key Features of the Gel Delivery System
Food Grade Ingredients
Our patent pending multiple-health ingredient dosage forms, our gel delivery technology and the ingredients delivered in our OTC, nutraceutical, sport and pet products are generally regarded as safe (“GRAS”), meaning ingredients used in these formulations have previously undergone safety evaluations by either a regulatory body (such as the FDA) or experts and have shown to not be harmful when used as intended. Our team, together with the assistance of our regulatory team, have reviewed each of our gel components, and given there is existing usages of the different compounds across different products, the Company is able to term the gel components as being GRAS.
We also do not make any health claims with respect to these products and therefore, we have concluded in consultation with our regulatory consultants that they can be marketed and sold with minimal regulatory oversight, which reduces lead times and costs, and makes it more suitable to a larger number of potential customers.
Transforming virtually any ingredient into a gel
Our gelification process makes it easy to transform any macronutrients, micronutrients, pharmaceutical or medicinal ingredients into a stand-alone gel product. We can gelify, or replace with a gel, a wide range of existing consumables, including powders, tablets, pills, supplements, vitamins, or oils, transforming them into, or replacing them with, a new
47
gel product. The gelification process involves a complex series of steps that allows us to form a gel matrix whereby ingredient(s) are homogeneously dispersed in the gel matrix and held in place, providing an easy to consume solution for consumers (human or animal).
Taste Masking
Taste masking is defined as a perceived reduction of an undesirable taste that would otherwise exist. The ideal solution to reduce or inhibit bitterness is the discovery of a universal inhibitor of all bitter tasting substances that does not affect the other taste modalities such as sweetness or saltiness. We regard most APIs as having an unpleasant or bitter taste, and Gelteq’s solutions were created to help moderate or mask unpleasant or bitter flavors without altering or damaging the taste receptors, and to ensure complete digestibility of the gel formulation, and thus have the potential to increase dosage compliance, palatability and commercial success.
Our scientists utilize a combination of taste assessment (meaning evaluation of a taste), taste moderation (meaning moderation of the extent to which an undesirable taste is perceived) and taste masking (meaning masking of an undesirable taste) to create palatable, customer-accepted forms of products for animal and human consumption.
Gelteq’s technology does not block taste receptors from working beyond consumption, which is hugely beneficial compared to alternatives developed by competitors which work on blunting receptors to mask taste. Our gel delivery system allows for the masking of taste by a method of encasing the nutrients and minimizing their release on certain taste receptor areas, which allows consumers to continue to taste their next mouthful unaffected by the masking product. In contrast, many taste masking products block out a taste reception for several hours which can change the user’s taste during the following meals and can have a negative impact on future consumption of the masking products.
Variety of textures — differing viscosities
Our gelification process is able to be customized across different textures. This allows us to work with clients across many different sectors including, but not limited to animals, children, seniors, or athletes.
The usefulness of our ability to control viscosities can be seen in helping conditions like dysphagia (the medical term given to difficulty swallowing) which will be discussed in more detail below.
Set dosage
While tablets or capsules do provide set dosages, many liquids require user preparation. This can lead to a high probability of user error, either under- or overdosing. Having a clear and defined dose in our gel dosage allows for accuracy and efficiency for the end users. This can also enhance compliance with the required dosage by users given the ease of use which does not require syringes or measuring cups to get the right dosage.
Pet Market Insights
Supplements for Pets
In terms of value, the companion pets segment dominated the market with a revenue share of over 45.0% in 20208. Companion pets are the most popular pets in the world, with an incredibly high adoption rate. According to the American Pet Products Association’s 2019 – 2020 National Pet Owners Survey, approximately 63.4 million households in the United States own a dog, with owners spending an average of approximately USD$58 per year on dog vitamins.
For instance, vitamins and supplements may be given to around one-third of companion pets and cats in the U.S. According to a 2006 study published in the Journal of the American Veterinary Medical Association, the most prevalent are multivitamins, supplements to assist arthritic joints, and fatty acids to minimize shedding and increase a coat’s gloss. Probiotics can be given to pets to help with gastrointestinal issues and antioxidants can be given to fight the consequences of aging, such as cognitive deterioration.
____________
8 See Grand View Research (2022) Companion Animal Medicine Market Report
48
COVID-19 has clearly raised awareness of the necessity of supporting immune health in a proactive manner. According to a survey reported on by the Kerry Group plc9 more than a quarter of dog and cat owners in the U.S. are concerned about their pets’ health as a result of COVID-19. Furthermore, approximately 69% of these concerned pet owners have explored using immune-strengthening supplements in their pet’s diet.
Pet Humanization
Globally, pet humanization has received a lot of attention in mainstream media over the recent past. The shift from pet ownership to pet parenting has been a very crucial and defining trend in the pet food market, more so in the developed countries. Over one-third of the households in the developed countries own a pet.10 According to the American Pet Products Association’s 2019 – 2020 National Pet Owners Survey, it revealed that more than 85 million households in the United States had one or more pets, the majority of them being companion pets. Thus, increasing pet humanization is anticipated to drive the pet food industry.
As a part of this pet humanization trend, pets are considered a part of the family. The growing bond between pet owners and their pets correlates with consumers’ willingness to spend more on pet food. Consumers are now becoming aware of their pet’s health and are buying pet food rich in nutritional value for the betterment of their companion pets. Nowadays, pet owners are not just looking for basic food products but also for pet consumables that are locally produced and natural or have specific health benefits.11
Additionally, the pet humanization trend has led to increased health consciousness and has generated demands for pet food free from sugar, grain, dye, and other chemical additives. Hence, with the emerging pet humanization and premiumization trends, the pet food demand is expected to grow further in the coming years.12
Companion Pet Health
Within the pet nutrition industry, pet supplements are often overshadowed by the excitement and innovation taking place in the pet food and treat categories.13 However, 2020 revealed a seismic shift and a burgeoning opportunity for pet supplement manufacturers.14
Unsurprisingly, new product development (“NPD”) within the North American pet nutrition market dropped by 28% in 2020 versus the prior three-year average, according to Innova,15 likely due to challenges from COVID-19. However, one rising development was pet supplements, which showed a staggering increase of approximately 116% growth from 2019 to 2020, with more than 150 NPD activities within the North American marketplace.
The billion-dollar pet supplement business in North America has historically been driven by joint health as well as skin and coat health, with a steady transition from brick-and-mortar purchases to online sales. However, COVID-19 disrupted trends in the pet product category, leading to a steep rise in immune system and digestive health products for pets and a dramatic shift to online purchasing.
Immune support is in-demand
COVID-19 undoubtedly has accelerated awareness of the importance of proactively supporting immune health. A survey of U.S. dog and cat owners conducted by Kerry found that more than a quarter report feeling more concerned about their pet’s health as a result of COVID-19, and approximately 69% of these concerned consumers have considered adding immune strength-supporting products to their pet’s diet. For consumers who have already taken steps to improve pet immunity through nutrition, approximately 38% turned to supplements. Pet supplement manufacturers were aware of this consumer trend as there were approximately 236% more immune health claims amongst pet supplement NPD in 2020 versus 2019.
____________
9 See Kerry (2022). Pet Wellness and Nutrition
10 See American Pet Products Association’s 2019 – 2020 National Pet Owners Survey.
11 See Pet Food Market — Growth, Trends, COVID-19 Impact, and Forecasts (2021 – 2026).
12 See Pet Food Market — Growth, Trends, COVID-19 Impact, and Forecasts (2021 – 2026)
13 See Kerry (2022). Pet Wellness and Nutrition
14 Ibid.
15 Ibid.
49
Notable immune health pet supplement trends in 2020 include novel ingredients like cannabidiol (commonly referred to as CBD oil), hemp oil, krill oil and silver.16 Appealing product forms such as nutrition bars and meal toppers and natural flavors such as peanut butter and banana can help solve palatability and pet acceptance challenges with administering supplements. As the pet supplement category continues to grow and new ingredients are introduced to the market, brands may see consumers seeking more specific ingredient claims or pet supplements with the branded immune health ingredients they already know and trust in their own food and beverages.
Digestive health takes hold
Digestive health pet supplement claims rose by approximately 173% in 2020 compared to 2019.17 Probiotics are the go-to pet health ingredient to support pet digestive health as they are generally understood and accepted by consumers in their own food and beverage.18 When asked about the functional pet ingredient attributes that matter most to U.S. pet owners regarding keeping pets healthy in the wake of COVID-19, probiotics ranked second, just behind immunity ingredients, further signaling their perceived link to pet health. Bacillus in particular have seen the most significant growth within this product category, with Innova reporting an approximate 41% compound annual growth rate (“CAGR”) from 2016 to 2019.19
As the humanization of pets continues to drive growth of the pet food, treat and supplement market, consumers are opting for the ingredients they know and trust in their own diets. Mintel recently reported that approximately 59% consumers are skeptical of health claims made on pet nutrition products.20 This can create an opportunity for pet supplement manufacturers to leverage branded digestive health ingredients, which provide consumers with a clear point of reference when browsing shelves and helps to deliver on transparency and build trust.21
Human Market Insights
Gels directly combat the problems associated with Dysphagia
Dysphagia, the medical term given to difficulty swallowing, can occur anatomically as oral dysphagia (in the mouth), pharyngeal dysphagia (in the pharynx itself), or cricopharyngeal dysphagia (at the far end of the pharynx entering the esophagus).
Oral dysphagia can be caused by paralysis of the jaw, tongue paralysis, dental disease, swelling or wasting away of the chewing muscles, or by an inability to open the mouth. Animals with oral dysphagia often eat in an altered way, such as tilting the head to one side or throwing the head backward while eating. Dysphagia can occur in humans for many reasons, most notably an underlying medical condition, post serious health event (for example, stroke) or can occur through the aging process through lost muscle tone. This is normally treated by adjusting the food and fluid textures depending on the level of swallowing difficulty and choking risk. Gelteq is currently focused on providing solutions to those suffering from dysphagia, with dogs being our first foray within animal health, followed later by humans.
As we continue to expand our gel solutions with dysphagia capabilities, Gelteq engaged with Monash University’s Medicines Manufacturing Innovation Centre (“MMIC”) to validate our technology for use in humans with dysphagia. The validation centered around analyzing our gel solution’s structure and functionality against the dysphagia standards, determining its suitability for use by humans with dysphagia. A white paper report was prepared at our request by MMIC in November 2021, which outlines MMIC’s assessment and expert opinion and concludes that “products manufactured with the Gelteq Delivery System can be designed to be homogeneous and have fluidity and texture directly useful in the management of dysphagia and swallowing difficulty as well as for the management of strong or unpleasant taste. The carrying capacity of the gel makes it suitable for the formulation of high payload products such as foods and nutrients for easy swallowing and portion management. The capacity of the gel is also useful for the management of appropriate pharmaceutical products either alone or as part of a combination treatment, polypharmacy, or co-administration of supplements, absorption aids, or other orally administered components.”22
____________
16 Ibid.
17 Ibid.
18 Ibid.
19 Ibid.
20 Ibid.
21 Ibid.
22 See Medicines Manufacturing Innovation Centre (2021), Delivery systems assisting the management of dysphagia, phagophobia, and swallowing aversion.
50
Nutraceuticals and Personalized Nutrition
Nutraceuticals are any substance that is a food or part of a food which provides medicinal or health benefits, including the prevention and treatment of disease. Nutraceuticals may be used to improve health, delay the aging process, prevent chronic diseases, increase life expectancy, or support the structure or function of the body.23 In recent years, nutraceuticals have received considerable interest due to potential nutritional, safety and therapeutic effects.24 Consumers are looking to fulfill nutrient and energy needs due to hectic work schedules. According to two of Grand View Research reports, all of this is driving an increase in spending on nutraceuticals. Nutraceuticals are expected to grow from approximately USD$140 billion in 2020 to USD$270 billion by 2028.
We plan to expand globally with our nutraceuticals & sports business partners who use Gelteq’s patent pending gel-based methods for delivery of multiple-health ingredients to develop gel pack dosage forms formulated with their ingredients.
As an example of a new license partner in the nutraceutical space, on July 1, 2021, we entered the U.S. market with a signed agreement of 500,000 units, with a Nevada based company, Healthy Extracts Inc. (OTCQB: HYEX) (“Healthy Extracts”), a leading innovator of clinically proven plant based products for heart and brain health. Gelteq formulated and created a new gel product for Healthy Extracts that is currently available for sale across the U.S. and Canada.
Sports
Compared with the general population, athletes are more likely to take ergogenic aids, which are dietary supplements marketed as enhancing endurance and/or strength, boosting exercise efficiency, increasing exercise tolerance, and attaining exercise goals more swiftly.25 Athletes, in particular elite athletes, use these supplements to prepare for exercise, help with recovery, and decrease chances of injury.
Athletes who want to ingest these supplements quickly and effortlessly, without bulking up on excess water, would benefit from a gel based delivery system.
Popular sports supplements which we are able to incorporate into our gel based delivery system include:
Branched-chain amino acids
The three branched-chain amino acids are leucine, isoleucine, and valine. Unlike other essential amino acids, these can be metabolized by mitochondria in skeletal muscle to yield energy for exercise. A small number of short-term clinical trials indicated that branched-chain amino acids might result in gains in muscle mass and strength during training.
Caffeine
This stimulant blocks activity of the sedative-like neuromodulator adenosine and decreases pain and perceived exertion. Clinical trials consistently support that when taken before physical activity, caffeine can improve performance, particularly in endurance activities, such as running, as well as in intermittent, long-duration activities like soccer.
Creatine
This supplement supplies muscles with energy for short, anaerobic bursts (for example., sprinting). A number of clinical trials support its benefit for high-intensity, intermittent activity, although these effects may vary by individual. Creatine has been shown in clinical trials to increase strength, work, and power for maximal-effort muscle contractions. Over time, it may aid athletes in adapting to training regimens. However, creatine’s benefits are negligible for endurance sports.
____________
23 See “New concepts in nutraceuticals as alternative for pharmaceuticals” by Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M in Int J Prev Med. 2014 December 5.
24 See Grand View Research, Sep. 2021 Industry Analysis Pet Supplements Market; Grand View Research, Jan 2021 Industry analysis Veterinary Medicine Market
25 See “10 — supplements — for — improved — athletic% performance” by Naveed Saleh. 2020 October 6
51
Glutamine
This amino acid contributes nitrogen to various biochemical reactions and is a key player in metabolism and energy production. Limited research has indicated that it may enhance recovery and/or muscle strength and decrease soreness post-exercise.
Iron
Iron boosts uptake of oxygen, lowers lactate levels during exercise, and decreases heart rate. Although clinical trials have shown mixed results, some evidence indicates that this essential mineral improves work capacity when correcting for anemia. However, it remains to be elucidated whether iron is ergogenic in people with milder anemia.
Protein
Protein provides essential amino acids to build, maintain, and repair muscle tissue. Based on a wide range of clinical data, protein enhances muscle training response during exercise and recovery. Many athletes take protein post-exercise, which is when it optimally reduces muscle protein breakdown, builds muscle, and enhances muscle oxygen use.
We can market our gel based products to companies who are looking to innovate in the sports nutrition space, offering them a distinctive advantage they can use against their competitors.
Oral drug delivery and diagnostics
The oral drug delivery market remains a huge part of the pharmaceutical industry. According to Data Bridge Market Research, the human oral drug delivery and diagnostics market is currently estimated at approximately USD$769 billion and, with a CAGR of approximately 6.9%, it is expected to grow to approximately USD$1,227 billion by 2027.
However, given its huge size, there has been relatively little innovation in how oral drugs are delivered, compared with the pace of innovation in other areas of health care. Liquid medicines date back to at least 4,000 B.C. and the use of pills to deliver medication can be traced to ancient Egypt to around 1,500 B.C. and the gelatin capsule was invented in around 1847.26 However, since then, innovation has been relatively modest.
As discussed in the next section, we believe that in our future collaborations with pharmaceutical companies, there may be potential patent life cycle management opportunities for difficult-to-deliver drugs and their new and improved dosage forms that can utilize our gel based delivery system.
Applications & Use Cases
Gelteq’s gel solution has numerous prospective applications across animal health, nutraceutical, pharmaceutical, over-the-counter healthcare and sport markets.
• Animal Health — Our gel formulations offer a potential solution for pets who have significant difficulties in swallowing pills, or simply as an alternative delivery vehicle to pills which can be a challenge to administer to any pet.
• Nutraceuticals — We have created various formulations that have the potential to enable the delivery of a large variety of macro or micronutrients for humans or animals, together with a large variety of nutraceutical ingredients.
• Pharmaceutical — Our gel delivery system has the potential to enable the delivery of pharmaceutical and medicinal ingredients, solving unmet pharmaceutical consumption issues around swallowing, taste, dosage and efficacy.
• Healthcare — The gel delivery system provides potential for effective, targeted, and flexible solutions within specialty healthcare areas, with core gel components such as viscosity, dose and release timing able to be tailored to service specific OTC drug requirements.
____________
26 See ““The Colorful History of Pills Can Fill Many a Tablet”. Los Angeles Times. Archived from the original on 19 September 2015”
52
• Sport Markets — Our gel delivery system provides potential to deliver key nutrients and minerals for improved sports performance, through our efficient and easy to consume gel delivery vehicle, which does not require additional water intake to gain the full benefit.
• Potential Patent Life Cycle management opportunities for difficult-to-deliver Drugs — We are seeking to file new patent applications based on improved combinations with custom-tailored versions of our drug delivery system to protect new dosage forms that we expect may arise. In addition to the pharmaceutical use case above, modified new versions of our gel-based delivery system that we seek to develop may allow drug companies to extend the patent life of their drugs by applying for a new patent insofar as new dosage forms were independently patentable. Such resulting downstream patent applications to advantageous combinations could extend a drug product’s patent life cycle with a new dosage form for the drug. This possibility can be extremely valuable for drug companies when they are near the loss of patent protection. It is estimated drugs with a total value of approximately USD$198 billion will have patents expire between 2019 and 2024 which we believe presents potential development opportunities for new improved delivery systems for which we believe patent protection may be available.
Research and Development
Our gel formulation has been formulated following extensive research into delivery methods across the pharmaceutical, over-the-counter healthcare, nutraceutical, sport and animal health markets, resulting in an oral delivery system that has the potential to serve a wide range of applications and consumers. Our research and current development in the financial year ending June 30, 2025 and onwards is conducted by our team of internal scientists and dietitians. We have previously also sort additional validation of our gel technology undertaken by MMIC to both verify and test our scientific methodologies. MMIC is one of the world’s leading drug discovery and global health research institutes in Australia which has previously analyzed each of our product created and, after conducting their lab-based tests, delivers reports on our product suite. We will continue with such processes with our new products with MMIC on an as-needed basis. Our gel delivery technology is food-based and is able to be used across food and medicine sectors for both humans and animals.
We are currently focused on further validating the gel technology and its capabilities within the veterinary space. We also aim to conduct clinical trials on an animal-based medication for the treatment of a chronic health condition. As part of our clinical research and development, we will also be conducting several animal and human trials to ensure we meet all compliance and registration requirements with the FDA on the Abbreviated New Animal Drug Application process (which is the animal equivalent pathway to the human drugs 505(b)(2) pathway).
Our next foray will be validating the gel technology for humans within the pharmaceutical space. Over the next 12-18 months we will be working with a multitude of pharmaceutical APIs that are available in different chemical structures. We will undertake a large amount of sampling and conduct lab-based tests to validate and test each of those products. Some examples of the tests that we will use are as follows:
• Release profile of active ingredient;
• Release times/comparisons;
• Drug load — max load;
• Extraction time frame;
• Viscosity level/viscosity ranges — in centipoise;
• Stability data;
• Bioequivalence study;
• Safety data; and
• PK tests.
These attributes will provide us with a suite of pharmaceutical products, showcasing the flexibility of our gel delivery technology.
53
With one of these off-patent APIs we are entering into the 505(b)(2) pathway, which has the potential to allow us to add a prescription product to our product portfolio that uses our gel base. This pathway will take an estimated 12 to 15 months, including lab-based testing and a series of clinical trials which are required to complete this process. As a part of our clinical research and development, animal and human clinical trials will be conducted. The initial clinical trials estimated to commence early in the financial year ending June 30, 2026. We have completed dissolution studies as part of the pre-clinical phase and are now in the process of designing the two clinical trials — initially one for animals followed by a human trial, both to showcase bioequivalence of this dosage form and its safety. Concurrently, shelf-life stability testing will be run by an FDA registered and inspected facility.
Material Contracts
There are a number of material contracts that are critical to the business, and initially these can be broken down by manufacturing, regulatory and sales.
Manufacturing Contracts
On August 7, 2021, we and Labixiaoxin (Fujian) Foods Industrial Co., Ltd. (“LaBi”), a large-scale Chinese gel manufacturer, entered into an Entrusted Processing Contract (the “LaBi Manufacturing Agreement”). LaBi provides Gelteq with a manufacturing solution for customers that require an ASEAN manufacturer and a lower cost base. LaBi maintains one of the largest snack food market shares in the People’s Republic of China, with particular strength coming from their jelly-based foods. LaBi is publicly listed on the Hong Kong Stock Exchange with nearly 1,500 employees, and manufacture more than 300 varieties of snack products which are exported to over 30 countries globally. The LaBi Manufacturing Agreement provides that upon us placing an order with LaBi, LaBi shall receive from us the sum of 70% of the total order amount after LaBi accepts such order and we agree to a proposed delivery date by LaBi. The remaining 30% shall be payable to LaBi before delivery of the order. The term of the agreement began on August 1, 2021 and ended on July 31, 2023, the agreement continued on a month-to-month basis. On November 19, 2024, we entered into a new agreement with similar terms with LaBi. The LaBi Manufacturing Agreement is terminable if either we or LaBi (i) violate the confidentiality clause of the agreement, (ii) engage in a serious breach of contract, (iii) enters into a bankruptcy or merger procedure or (iv) lose the ability to perform the contract due to deterioration of financial or business conditions. In connection with the LaBi Manufacturing Agreement, we and LaBi entered into a license agreement, dated August 24, 2021, whereby we agreed to license certain intellectual property rights to LaBi, solely for the purpose of executing our manufacturing orders.
On January 31, 2022, we and Wasatch Product Development LLC (“Wasatch”), a large-scale U.S based gel manufacturer, entered into a Contract Manufacturing Agreement (the “Wasatch Manufacturing Agreement”). Wasatch is responsible for manufacturing and conducting all steps of production and quality control for our nutraceutical and OTC products in North America. Wasatch is a full service, turn-key contract manufacturer specializing in high-end personal care, cosmetic, dental care, OTC, dietary supplement, and food products in bottles, tubes and flexible packaging. Wasatch is wholly owned by a global dietary supplements company which is listed on the NYSE. Wasatch employs over 500 employees and has over 250,000 square feet of manufacturing and warehouse space. Wasatch also runs state-of-the-art clean rooms, batching equipment, packaging lines, and post-fill treatments to provide unprecedented process control and product quality. Wasatch is an FDA registered OTC Manufacturer, cGMP, Medical Device Facility, Cosmetic Manufacturer, Food Facility and ISO 22716 certified. Wasatch is responsible for manufacturing and conducting all steps for production and quality controls of any of our nutraceutical and OTC products in North America. The Wasatch Manufacturing Agreement provides that, upon us placing a purchase order with Wasatch, we shall pay a per unit fee as set forth in the agreement. For each purchase order, Wasatch shall also present Gelteq an invoice for one-half of the total purchase order amount as a non-refundable deposit. The term of the Wasatch Manufacturing agreement began on January 31, 2021 for a period of two years. Unless we or Wasatch provides the other party 180 day written notice to terminate the agreement, at the end of the term, the Wasatch Manufacturing Agreement will automatically renew for a period of one year. Neither party provided the required notice to terminate and the Wasatch Manufacturing Agreement has automatically been extended for a further twelve months. The Wasatch Manufacturing Agreement is terminable if either we or Wasatch (i) provides the other party 3 months written notice to terminate or (ii) commits serious or persistent breaches of any provisions of the agreement.
54
Regulatory Contracts
On December 5, 2019, we, under our former name MyHypho Pty Ltd, entered into a Master Research Services Agreement (the “Monash MRSA”) with Monash University’s Medicines Manufacturing Innovation Center (“MMIC”). MMIC is responsible for testing and validating — sampling, trials and lab tests our product formulations and will assist the business in performing bioequivalence and clinical studies to obtain the relevant formal approvals. In the Monash MRSA, we may engage MMIC to provide research services as described in a statement of work, and MMIC shall receive a yearly fixed-fee, pro-rated as necessary, for each research officer assigned full-time to a statement of work. On May 15, 2021, we and MMIC entered into a Variation Agreement which further extended the term of the Monash MRSA until January 31, 2023 and modified the services rate for research officers. The Company and MMIC had further extended the terms of the Monash MRSA until June 30, 2023. Both parties remain in discussions for a new agreement to be entered into when Gelteq require MMIC’s services.
On November 1, 2021, we and Adjutor Healthcare Pty Ltd (“Adjutor”), a leading regulatory affairs consulting company, entered into a Master Services Agreement (the “Adjutor MSA”), to work toward obtaining all regulatory approvals necessary for the commercialization of our drug based gel product. Adjutor will manage all regulatory activities necessary, including conducting the legal and regulatory review process and carrying out the regulatory filings to obtain marketing approval in the United States. The Adjutor MSA provides that we may retain Adjutor to provide consulting services to be described in a statement of work. The term of the Adjutor MSA commenced on November 1, 2021 and will continue until terminated by either party under the provisions of the agreement. We may terminate the Adjutor MSA, or any statement of work entered under it, by providing 30 days written notice to Adjutor. Further, we or Adjutor may terminate the Adjutor MSA, by written notice to the other party, at anytime (i) the other party commits a breach of the Adjutor MSA and fails to remedy that breach within 10 business days of receiving a notice specifying that breach, (ii) the other party becomes insolvent or (iii) continued association with the other party is reasonably deemed likely to result in reputation damage.
Sales Contracts
On September 6, 2021, we and Sosna & Co, Inc. (“Sosna”), an outsourced sales distribution company with offices in New York, Toronto, Montreal and Calgary, entered a Consulting Agreement (the “Sosna Consulting Agreement”). Sosna has been engaged to represent us across North America for pharmaceutical projects. Sosna will utilize their existing networks to sign up a series of pharma projects for us and also launch nutraceutical partnerships for us. Sosna is a team of life sciences experts with more than 42 years of experience creating strategic partnerships. Sosna’s industry connections provide insight on trends and allow them to strategically leverage information on behalf of our clients. Sosna’s specialist sales consultants in the pharmaceutical and nutraceutical industries work in life sciences sales and distribution across North America. Sosna has been responsible for generating numerous pharma deals over the past 3 years. The Sosna Consulting Agreement provides that we shall pay Sosna a monthly fee of (i) USD$8,500 per month (unless waived) and (ii) 5% of the aggregate deal value from any secured new business transactions, subject to a maximum success fee of USD$1,000,000. The Sosna Consulting Contract continues on a month-to-month basis from September 6, 2022 unless terminated by 30 days written notice by either us or Sosna.
On December 2, 2024, we entered into an agreement with WPIC Marketing and Technologies Limited (“WPIC”) to assist with sales and distribution of our SportsGel products throughout the Asian Pacific region, commencing with China initially in March 2025. As of the date of this prospectus, we have four online stores across various platforms open in China as WPIC’s first region of focus.
Customer Contracts
We have entered into separate licensing agreements with seven licensees who are the first to perform a sales trial and sell the products to their respective customers and chosen markets. Each licensing agreement comes with a corresponding order, and to date, we have over one million units ordered as part of these deals. We believe this pipeline will generate a further revenue which would improve our financial position. We have already delivered part of this pipeline, receiving revenue during the year ended June 30, 2023 and year ended June 30, 2022, with no recognized revenue for the six months ended December 31, 2024. The orders (license agreements) are for a range of gel products across the sport and nutraceutical verticals, and are a combination of our existing white label products, along with newly created private label products. Agreements and orders have also been placed from multiple countries; most notably Australia, the People’s Republic of China, and the United States. No regulatory approvals are believed to be required on any of these orders as all have been classified as food-based products with no medical claims being made.
55
Competition
A number of companies in the pharmaceutical market which have novel and innovative drug delivery systems in the pipeline such as transdermal patches, oral films, injection, and chewing gum. Among these companies are Oramed Pharmaceuticals, Inc. (NASDAQ: ORMP), IntelGenx Technologies Corp. (OTCMKTS: IGXT), BioDelivery Sciences International Inc. (NASDAQ: BDSI), Lexaria Bioscience Corp. (NASDAQ: LEXX), Taro Pharmaceuticals Industries Ltd. (NYSE: TARO), Catalent Inc. (NYSE: CTLT), Insulet Corporation (NASDAQ: CTLT), Nutriband Inc. (NASDAQ: NTRB), Virpax Pharmaceuticals Inc. (NASDAQ: VRPX) and Hempfusion Wellness Inc. (TSE: CBD.U). Despite the number of competitors, our gel delivery system is unique within the pharmaceutical space, we are not aware of any companies currently offering drug delivery in a similar gel base as at the date of this prospectus. For our products to receive FDA approval, we will have to demonstrate its efficacy, safety and ease of use provides an attractive alternative to existing delivery mediums, some of which are widely recognized and accepted by physicians and patients. Many of the competitors within the pharmaceutical market have substantially greater financial, technical and human resources than we do. We rely on our intellectual property and the strong partnerships we have with manufacturers and suppliers, to develop and provide superior products that use our gel delivery technology and patent pending multiple-health ingredient gel dosage forms.
The oral drug industry is subject to heavy competition and a rising demand for innovative oral solutions beyond traditional methods such as pills, syrups, capsules, drops, powders and gummies. Our ability to compete is based on a variety of factors, including product efficacy, bioequivalence, safety, patient compliance and ease of use.
Marketing and Sales
Our core marketing strategy is centered around signing up new license partners and distributors. We will actively be searching for new license partners and distributors across different verticals where there is an opportunity to either white label an existing Gelteq formulation, or to create a bespoke private label gel product for a particular market.
We have identified license partners and distributors as the quickest and most lucrative path to commercialization. All licensees already have existing clients with a pre-existing brand presence. By launching a new gel product into an already existing ecosystem, we believe the adoption rate will be higher and faster than creating our own products and launching them into a new market.
To grow our sales, we will use internal sales staff to identify, sell and promote our product to potential licensees. Initially, we may set up sales offices and representation in territories with potential interest in our products, such as the United States, Canada, the People’s Republic of China, Hong Kong, Australia, New Zealand, Malaysia or the United Kingdom.
We also plan to further utilize specialist sales consultants in the pharmaceutical and nutraceutical industries to act as referral partners and ongoing business development advisors. They will utilize their existing networks to sign up a series of pharma projects and also launch nutraceutical partnerships. An example of this can be highlighted by our partnership with Sosna. They are responsible for generating numerous pharma deals over the past 6 years and they are now engaged to represent Gelteq across North America for pharmaceutical projects.
A license partner or distributor could have multiple gel products, and thus, in effect, it could become a client for multiple products. We have examples of existing clients who have created multiple gel products with Gelteq, creating a higher overall total transaction value with the client, meaning total fair market value of the transactions with the client.
In addition to the above marketing methods, expects to continue to be present at many conferences, trade shows and summits as it will use these public forums as the foundation to meet with potential new license partners and distributors.
Manufacturing
We rely on and expect to continue to rely on third-party contract manufacturing organizations, or CMOs, for the supply of current good manufacturing practice-grade, or cGMP-grade, clinical trial materials and commercial quantities of our product candidates and products, if approved. We currently do not have any agreements for the commercial production of raw materials we use. We believe that the manufacturing process for the raw materials we purchase can be transferred to a number of other CMOs for the production of clinical and commercial supplies of our product candidates in the ordinary course of business.
56
At present, Gelteq products are manufactured by two production facilities: a production facility located in Draper, Utah, that is owned by a US-based comprehensive product manufacturing company, and a factory owned by a Chinese-based food industry company located in Quanzhou, the People’s Republic of China.
The US-based comprehensive product manufacturing company employs over 500 employees working on 19 production lines in two facilities with over 250,000 square feet of manufacturing and warehouse space. This company’s state-of-the-art clean rooms, batching equipment, packaging lines, and post-fill treatments provide unprecedented process control and product quality.
The Chinese-based food industry company maintains the second largest snack food market share in the People’s Republic of China, with particular strength coming from their jelly-based foods. Listed on the Hong Kong Stock Exchange with nearly 1,500 employees, it manufactures more than 300 varieties of snack products which are exported to over 30 countries globally.
Quality Control
We are committed to the highest quality of products that leave our facilities. To that end, we have implemented a rigid quality control system and devote significant attention to quality control procedures at every stage of our process, including spot testing of finished products. Our entire supply processing chain, from sourcing of raw materials to the finished products, is closely monitored to ensure that all products meet the highest level of global hygienic and quality standards. We monitor our manufacturing process closely and conduct performance and reliability testing to ensure our products meet our end-user customer expectations. We spot test and inspect our raw materials to ensure compliance with quality standards. We also evaluate the quality and delivery performance of each supplier periodically and adjust quantity allocations accordingly. We also monitor in-process and outgoing stages of our processes.
We have established control points throughout the entire supply chain from ingredient sourcing to finished goods to ensure compliance with our quality program. We require our contract and owned manufacturing facilities to maintain the same quality standards as those at our facilities and pass our own quality system and ingredient safety inspections. We ensure that all of our ingredients are rigorously tested prior to being approved for use in our products. Testing certifications which confirm that the ingredient meets our specifications as to quality and safety, accompany every shipment. In addition, our food safety and quality program include strict guidelines for incoming ingredients, batching, processing, packaging and finished goods.
Quality Certifications and Accreditations
In a continuous effort to meet various international production and quality manufacturing standards, we only work with parties who have secured certifications and accreditations that prove high quality standards. We utilize high-quality manufacturing standards and apply these to our production and management processes for domestic and foreign markets. We believe that maintaining objectively verifiable quality standards fosters consumer confidence and loyalty, and maximizes customer satisfaction and recognition.
Government Regulations
Our business is subject to extensive government regulation. Regulation by governmental authorities in the United States and other jurisdictions is a significant factor in the research, development, manufacture and commercialization of our product candidates and in our ongoing research and development activities.
Product Approval Process in the United States
Review and approval of drugs
In the United States, pharmaceutical products are subject to extensive regulation by the FDA. Note that health supplements, such as vitamins and nutraceuticals, are regulated by the FDA as food, not as drugs, and therefore are not subject to clinical trials and other investigations.
The Federal Food, Drug, and Cosmetic Act (FDCA) and other federal and state statutes and implementing regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and
57
export of products. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval may subject an applicant to a variety of administrative or judicial sanctions and enforcement actions brought by the FDA, the Department of Justice or other governmental entities. Possible sanctions may include the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties and other federal and state statutes and implementing regulations govern, among other things, the research, development, testing, manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting, sampling, and import and export of products. Failure to comply with the applicable U.S. requirements at any time during the product research and development process, approval process or after approval may subject an applicant to a variety of administrative or judicial sanctions and enforcement actions brought by the FDA, the Department of Justice or other governmental entities. Possible sanctions may include the FDA’s refusal to approve pending applications, withdrawal of an approval, imposition of a clinical hold, issuance of warning letters or untitled letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement and civil or criminal penalties.
FDA approval of a new drug application is required before any new unapproved drug or dosage form can be marketed in the United States. Section 505 of the FDCA describes three types of new drug applications: (1) an application that contains full reports of investigations of safety and effectiveness (section 505(b)(1)); (2) an application that contains full reports of investigations of safety and effectiveness but where at least some of the information required for approval comes from studies not conducted by or for the applicant and for which the applicant has not obtained a right of reference (section 505(b)(2)); and (3) an application that contains information to show that the proposed product is identical in active ingredient, dosage form, strength, route of administration, labeling, quality, performance characteristics, and intended use, among other things, to a previously approved product (section 505(j)). Section 505(b)(1) and 505(b)(2) new drug applications are referred to as NDAs, and section 505(j) applications are referred to as ANDAs.
In general, the process required by the FDA prior to marketing and distributing a new drug, as opposed to a generic drug subject to section 505(j), in the United States usually involves the following:
• completion of pre-clinical laboratory tests, animal studies and formulation studies in compliance with the FDA’s good laboratory practices, or GLP, requirements or other applicable regulations;
• submission to the FDA of an investigational new drug application, or IND, which must become effective before human clinical trials in the United States may begin;
• approval by an independent institutional review board, or IRB, at each clinical site before each trial may be initiated;
• performance of adequate and well-controlled human clinical trials in accordance with good clinical practice, or GCP, requirements to establish the safety and efficacy of the proposed drug for its intended use;
• preparation and submission to the FDA of an NDA;
• satisfactory completion of an FDA advisory committee review, if applicable;
• satisfactory completion of one or more FDA inspections of the manufacturing facility or facilities at which the product or components thereof are produced, to assess compliance with current good manufacturing practices, or cGMPs, and to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity;
• satisfactory completion of FDA audits of clinical trial sites to assure compliance with GCPs and the integrity of the clinical data;
• payment of user fees and FDA review and approval of the NDA; and
• compliance with any post-approval requirements, including the potential requirement to implement a Risk Evaluation and Mitigation Strategy, or REMS, and the potential requirement to conduct post-approval studies.
58
Preclinical studies
Preclinical studies include laboratory evaluation or product chemistry, formulation and toxicity, as well as animal studies to assess the potential safety and efficacy of the product candidate. Pre-clinical safety tests must be conducted in compliance with the FDA regulations. The results of the preclinical studies, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND which must become effective before clinical trials may commence. Long-term pre-clinical studies, such as animal tests of reproductive toxicity and carcinogenicity, may continue after the IND application is submitted.
Clinical trials
Clinical trials involve the administration of an investigational product to human subjects under the supervision of qualified investigators in accordance with GCP requirements, which include, among other things, the requirement that all research subjects provide their informed consent in writing before their participation in any clinical trial. Clinical trials are conducted under written trial protocols detailing, among other things, the objectives of the trial, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND.
An IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions related to a proposed clinical trial and places the trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. In addition, information about certain clinical trials must be submitted within specific timeframes to the National Institutes of Health, or NIH, for public dissemination on the NIH-maintained website, www.clinicaltrials.gov.
An IRB representing each institution participating in the clinical trial must review and approve the plan for any clinical trial before it commences at that institution, and the IRB must conduct continuing review at least annually. The IRB must review and approve, among other things, the trial protocol information to be provided to trial subjects. An IRB must operate in compliance with FDA regulations. Information about certain clinical trials must be submitted within specific timeframes to the NIH for public dissemination on the NIH-maintained website, www.clinicaltrials.gov.
Clinical trials are typically conducted in three sequential phases, which may overlap or be combined:
• Phase I: The drug is initially introduced into healthy human subjects or patients with the target disease or condition and tested for safety, dosage tolerance, absorption, metabolism, distribution, excretion and, if possible, to gain an early indication of its effectiveness and to determine optimal dosage.
• Phase II: The drug is administered to a limited patient population to identify possible short-term adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage.
• Phase III: The drug is administered to an expanded patient population, generally at geographically dispersed clinical trial sites, in well-controlled clinical trials to generate enough data to statistically evaluate the efficacy and safety of the product for approval, to establish the overall risk-benefit profile of the product, and to provide adequate information for the labeling of the product.
In most cases of an ANDA, the proposed generic drug must be shown to be bioequivalent to the reference listed drug (RLD, or reference product) and in other cases, the bioequivalent study is being conducted in in-vitro and not in clinical trials. The FDCA provides that a generic drug is bioequivalent to the listed drug if: the rate and extent of absorption of the drug do not show a significant difference from the rate and extent of absorption of the listed drug when administered at the same molar dose of the therapeutic ingredient under similar experimental conditions in either a single dose or multiple doses. During bioequivalence studies, an applicant compares the systemic exposure profile of a test drug product to that of the RLD on the target population at the same regimen and exposure period as the RLD where the resulting efficacy outcomes are compared to demonstrate being equivalent.
Submission of an NDA to the FDA
The results of the pre-clinical studies and clinical trials, together with other detailed information, including information on the manufacture, control and composition of the product, are submitted to the FDA as part of an NDA requesting approval to market the product candidate for a proposed indication. Under the Prescription Drug User Fee Act, as
59
amended, applicants are required to pay fees to the FDA for reviewing an NDA. These user fees, as well as the annual fees required for commercial manufacturing establishments and for approved products, can be substantial. The NDA review fee alone can exceed USD$2 million, subject to certain limited deferrals, waivers and reductions that may be available.
The FDA has 60 days from its receipt of an NDA to determine whether the application will be accepted for filing based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. The FDA may request additional information rather than accept an NDA for filing. In this event, the NDA must be resubmitted with the additional information and is subject to payment of additional user fees. The resubmitted application is also subject to review before the FDA accepts it for filing. If found complete, the FDA will accept the NDA for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review.
Under the Prescription Drug User Fee Act, the FDA has agreed to certain performance goals in the review of NDAs through a two-tiered classification system, Standard Review and Priority Review. Review. Priority Review designation is given to drugs that offer major advances in treatment, or provide a treatment where no adequate therapy exists. The FDA endeavors to review applications subject to Standard Review within approximately 10 to 12 months of receipt, whereas the FDA’s goal is to review Priority Review applications within approximately six to eight months of receipt, depending on whether the drug is a new molecular entity. The FDA, however, may not approve a drug within these established goals, and its review goals are subject to change from time to time.
Before approving an NDA, the FDA inspects the facilities at which the product is manufactured or facilities that are significantly involved in the product development and distribution process, and will not approve the product unless cGMP compliance is satisfactory. Additionally, the FDA will typically inspect one or more clinical sites to assure compliance with GCP requirements. The FDA may also refer applications for novel drug products or drug products which present difficult questions of safety or efficacy to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions.
After the FDA evaluates the NDA and the manufacturing facilities, it issues either an approval letter or a complete response letter to indicate that the review cycle for an application is complete and that the application is not ready for approval. A complete response letter generally outlines the deficiencies in the submission and may require substantial additional testing, or information, in order for the FDA to reconsider the application. Even with submission of this additional information, the FDA may ultimately decide that an application does not satisfy the regulatory criteria for approval. If, or when, the deficiencies have been addressed to the FDA’s satisfaction in a resubmission of the NDA, the FDA will issue an approval letter. An approval letter authorizes commercial marketing of the drug with specific prescribing information for specific indications.
If a product is approved, the approval will impose limitations on the indicated uses for which the product may be marketed, may require that warning statements be included in the product labeling, may require that additional studies or trials be conducted following approval as a condition of the approval, may impose restrictions and conditions on product distribution, prescribing or dispensing in the form of a risk management plan, or impose other limitations. For example, as a condition of NDA approval, the FDA may require a risk evaluation and mitigation strategy, or REMS, to ensure that the benefits of the drug outweigh the potential risks. If the FDA determines a REMS is necessary during review of the application, the drug sponsor must agree to the REMS plan at the time of approval. A REMS may be required to include various elements, such as a medication guide or patient package insert, a communication plan to educate healthcare providers of the drug’s risks, limitations on who may prescribe or dispense the drug, or other elements to assure safe use, such as special training or certification for prescribing or dispensing, dispensing only under certain circumstances, special monitoring and the use of patient registries. In addition, the REMS must include a timetable to periodically assess the strategy. The requirement for a REMS can materially affect the potential market and profitability of a drug.
Further changes to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes or facilities, require submission and FDA approval of a new NDA or NDA supplement before the change can be implemented, which may require us to develop additional data or conduct additional pre-clinical studies and clinical trials. An NDA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA uses the similar procedures in reviewing NDA supplements as it does in reviewing NDAs.
60
Post-Approval Requirements
Any drug products for which we receive FDA approval will be subject to continuing regulation by the FDA. Certain requirements include, among other things, record-keeping requirements, reporting of adverse experiences with the product, providing the FDA with updated safety and efficacy information on an annual basis or more frequently for specific events, product sampling and distribution requirements, complying with certain electronic records and signature requirements and complying with FDA promotion and advertising requirements. These promotion and advertising requirements include standards for direct-to-consumer advertising, prohibitions against promoting drugs for uses or patient populations that are not described in the drug’s approved labeling, known as “off-label use,” and other promotional activities, such as those considered to be false or misleading. Failure to comply with FDA requirements can have negative consequences, including the immediate discontinuation of non-complying materials, adverse publicity, enforcement letters from the FDA, mandated corrective advertising or communications with doctors, and civil or criminal penalties. Such enforcement may also lead to scrutiny and enforcement by other government and regulatory bodies.
Although physicians may prescribe legally available drugs for off-label uses, manufacturers may not encourage, market or promote such off-label uses. As a result, “off-label promotion” has formed the basis for litigation under the Federal False Claims Act, violations of which are subject to significant civil fines and penalties. In addition, manufacturers of prescription products are required to disclose annually to the Center for Medicaid and Medicare any payments made to physicians in the United States under the Sunshine Act of 2012. These payments could be in cash or kind, could be for any reason, and are required to be disclosed even if the payments are not related to the approved product. A failure to fully disclose or not report in time could lead to penalties of up to USD$1 million per year.
The manufacturing of any of our product candidates will be required to comply with applicable FDA manufacturing requirements contained in the FDA’s cGMP regulations. The FDA’s cGMP regulations require, among other things, quality control and quality assurance, as well as the corresponding maintenance of comprehensive records and documentation. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are also required to register their establishments and list any products they make with the FDA and to comply with related requirements in certain states. Changes to the manufacturing process are strictly regulated and often require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMP requirements and impose reporting and documentation requirements upon the sponsor and any third-party manufacturers that the sponsor may decide to use. These entities are further subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain cGMP compliance.
Discovery of problems with a product after approval may result in serious and extensive restrictions on a product, manufacturer or holder of an approved NDA, as well as lead to potential market disruptions. These restrictions may include recalls, suspension of a product until the FDA is assured that quality standards can be met, and continuing oversight of manufacturing by the FDA under a “consent decree,” which frequently includes the imposition of costs and continuing inspections over a period of many years, as well as possible withdrawal of the product from the market. In addition, changes to the manufacturing process generally require prior FDA approval before being implemented. Other types of changes to the approved product, such as adding new indications and additional labeling claims, are also subject to further FDA review and approval. There also are continuing, annual user fee requirements for any marketed products and the establishments at which such products are manufactured, as well as new application fees for supplemental applications with clinical data.
The FDA also may require post-marketing testing, or Phase IV testing, as well as risk minimization action plans and surveillance to monitor the effects of an approved product or place conditions on an approval that could otherwise restrict the distribution or use of our product candidates.
Once approval is granted, the FDA may withdraw the approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in mandatory revisions to the approved
61
labeling to add new safety information; imposition of post-market studies or clinical trials to assess new safety risks; or imposition of distribution or other restrictions under a REMS program. Other potential consequences include, among other things:
• restrictions on the marketing or manufacturing of the product, complete withdrawal of the product from the market or product recalls;
• fines, warning letters or holds on post-approval clinical trials;
• refusal of the FDA to approve pending NDAs or supplements to approved NDAs, or suspension or revocation of product approvals;
• product seizure or detention, or refusal to permit the import or export of products; or
• injunctions or the imposition of civil or criminal penalties.
Pediatric trials and exclusivity
Even when not pursuing a pediatric indication, under the Pediatric Research Equity Act of 2003, an NDA or supplement thereto must contain data that is adequate to assess the safety and effectiveness of the drug product for the claimed indications in all relevant pediatric subpopulations, and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. With the enactment of the Food and Drug Administration Safety and Innovation Act, or the FDASIA, in 2012, sponsors must also submit pediatric trial plans prior to the assessment data. Those plans must contain an outline of the proposed pediatric trials the applicant plans to conduct, including trial objectives and design, any deferral or waiver requests, and other information required by regulation. The applicant, the FDA, and the FDA’s internal review committee must then review the information submitted, consult with each other, and agree upon a final plan. The FDA or the applicant may request an amendment to the plan at any time.
The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Additional requirements and procedures relating to deferral requests and requests for extension of deferrals are contained in the FDASIA.
Separately, in the event the FDA makes a written request for pediatric data relating to a drug product, an NDA sponsor who submits such data may be entitled to pediatric exclusivity. Pediatric exclusivity is another type of non-patent marketing exclusivity in the United States and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing exclusivity.
The Hatch-Waxman Amendments
ANDA Approval Process
The Drug Price Competition and Patent Term Restoration Act of 1984 (also known as the Hatch-Waxman Amendments), established abbreviated FDA approval procedures for drugs that are shown to be equivalent to proprietary drugs previously approved by the FDA through its NDA process. Approval to market and distribute these drugs is obtained by submitting an ANDA with the FDA. An ANDA is a comprehensive submission that contains, among other things, data and information pertaining to the active pharmaceutical ingredient, drug product formulation, specifications and stability of the generic drug, as well as analytical methods, manufacturing process validation data, and quality control procedures. Premarket applications for generic drugs are termed abbreviated because they generally do not include pre-clinical and clinical data to demonstrate safety and effectiveness. Instead, a generic applicant must demonstrate that its product is bioequivalent to the innovator drug. In certain situations, an applicant may obtain ANDA approval of a generic product with a strength or dosage form that differs from a referenced innovator drug pursuant to the filing and approval of an ANDA Suitability Petition. The FDA will approve the generic product as suitable for an ANDA application if it finds that the generic product does not raise new questions of safety and effectiveness as compared to the innovator product. A product is not eligible for ANDA approval if the FDA determines that it is not bioequivalent to the referenced innovator drug, if it is intended for a different use, or if it is not subject to an approved Suitability Petition. However, such a product might be approved under an NDA, with supportive data from clinical trials.
62
505(b)(2) NDAs
Section 505(b)(2) was enacted as part of the Hatch-Waxman Amendment, and permits the filing of an NDA where at least some of the information required for approval comes from studies or trials not conducted by or for the applicant and for which the applicant has not obtained a right of reference. As an alternative path to FDA approval for modifications to formulations or uses of products previously approved by the FDA, an applicant may submit an NDA under Section 505(b)(2) of the FDCA. If the 505(b)(2) applicant can establish that reliance on the FDA’s previous findings of safety and effectiveness is scientifically appropriate, it may eliminate the need to conduct certain pre-clinical studies or clinical trials for the new product. The FDA may also require companies to perform additional studies or measurements, including clinical trials, to support the change from the approved branded reference drug. The FDA may then approve the new product candidate for all, or some, of the labeled indications for which the branded reference drug has been approved, as well as for any new indication sought by the 505(b)(2) applicant.
Orange Book Listing
In seeking approval for a drug through an NDA, including a 505(b)(2) NDA, applicants are required to list with the FDA certain patents whose claims cover the applicant’s product. Upon approval of an NDA, each of the patents listed in the application for the drug is then published in the FDA’s Publication of Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the “Orange Book.” Any applicant who submits an ANDA seeking approval of a generic equivalent of a drug listed in the Orange Book or a Section 505(b)(2) NDA referencing a drug listed in the Orange Book must certify to the FDA that (1) no patent information on the drug product that is the subject of the application has been submitted to the FDA; (2) such patent has expired; (3) the date on which such patent expires; or (4) such patent is invalid or will not be infringed upon by the manufacture, use, or sale of the drug product for which the application is submitted. This last certification is known as a Paragraph IV certification. The applicant may also elect to submit a “section viii” statement certifying that its proposed label does not contain (or carves out) any language regarding the patented method-of-use rather than certify to a listed method-of-use patent.
If the applicant does not challenge one or more listed patents through a Paragraph IV certification, the FDA will not approve the ANDA or Section 505(b)(2) NDA until all the listed patents claiming the referenced product have expired. Further, the FDA will also not approve, as applicable, an ANDA or Section 505(b)(2) NDA until any non-patent exclusivity, as described in greater detail below, has expired.
If the ANDA or Section 505(b)(2) NDA applicant has provided a Paragraph IV certification to the FDA, the applicant must also send notice of the Paragraph IV certification to the owner of the referenced NDA for the previously approved product and relevant patent holders within 20 days after the ANDA or Section 505(b)(2) NDA has been accepted for filing by the FDA. The NDA and patent holders may then initiate a patent infringement suit against the ANDA or Section 505(b)(2) applicant. Under the FDCA, the filing of a patent infringement lawsuit within 45 days of receipt of the notification regarding a Paragraph IV certification automatically prevents the FDA from approving the ANDA or Section 505(b)(2) NDA until the earliest to occur of 30 months beginning on the date the patent holder receives notice, expiration of the patent, settlement of the lawsuit, or until a court deems the patent unenforceable, invalid or not infringed. Even if a patent infringement claim is not brought within the 45-day period, a patent infringement claim may be brought under traditional patent law, but it does not invoke the 30-month stay.
Moreover, in cases where an ANDA or Section 505(b)(2) application containing a Paragraph IV certification is submitted after the fourth year of a previously approved drug’s five-year NCE exclusivity period, as described more fully below, and the patent holder brings suit within 45 days of notice of the Paragraph IV certification, the 30-month period is automatically extended to prevent approval of the Section 505(b)(2) application until the date that is seven and one-half years after approval of the previously approved reference product that has the five-year NCE exclusivity. The court also has the ability to shorten or lengthen either the 30 month or the seven and one-half year period if either party is found not to be reasonably cooperating in expediting the litigation.
Notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), over the last few years, some pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). In addition to patent exclusivity, the holder of the NDA for the listed drug may be entitled to a period of non-patent exclusivity, during which the FDA cannot approve an ANDA or 505(b)(2) application that relies on the listed drug. For example, a pharmaceutical manufacturer may obtain five years of non-patent exclusivity upon NDA approval of a new chemical entity, or NCE, which is a drug that contains an active moiety that has not been approved by FDA in any other NDA. An ‘‘active moiety’’ is defined as the molecule or ion responsible for the drug substance’s physiological
63
or pharmacologic action. During the five year exclusivity period, the FDA cannot accept for filing any ANDA seeking approval of a generic version of that drug or any 505(b)(2) NDA for the same active moiety and that relies on the FDA’s findings regarding that drug, except that FDA may accept an application for filing after four years if the follow-on applicant makes a paragraph IV certification.
Another form of non-patent exclusivity is clinical investigation exclusivity. A drug, including one approved under Section 505(b)(2), may obtain a three-year period of exclusivity for a particular condition of approval, or change to a marketed product, such as a new formulation for a previously approved product, if one or more new clinical trials (other than bioavailability or bioequivalence studies) was essential to the approval of the application and was conducted/sponsored by the applicant. Should this occur, the FDA would be precluded from approving any ANDA or 505(b)(2) application for the protected modification until after that three-year exclusivity period has run. However, unlike NCE exclusivity, the FDA can accept an application and begin the review process during the exclusivity period.
Patent Term Restoration and Extension
A patent claiming a new drug product may be eligible for a limited patent term extension under the Hatch-Waxman Act, or PTE, which permits an extended patent term of up to five years for the developed pharmaceutical to compensate for patent term lost during product development and the FDA regulatory review. The PTE period granted is typically one-half the time between the effective date of an IND and the submission date of an NDA, plus the time between the submission date of an NDA and the ultimate approval date. However, the PTE cannot be used to extend the remaining term of a patent past a total of 14 years from the product’s approval date. Only one patent applicable to an approved drug product is eligible for the extension, and the application for the extension must be submitted prior to the expiration of the patent in question. A patent that covers multiple drugs for which approval is sought can only be extended in connection with one of the approvals. The U.S. Patent and Trademark Office reviews and approves the PTE application in consultation with the FDA.
Review and Approval of Drug Products Outside the United States
In addition to regulations in the United States, if we target non-U.S. markets, we will be subject to a variety of foreign regulations governing manufacturing, clinical trials, commercial sales and distribution of our future product candidates. Whether or not we obtain FDA approval for a product candidate, we must obtain approval of the product by the comparable regulatory authorities of foreign countries before commencing clinical trials or marketing in those countries. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from country to country.
Under European Union regulatory systems, marketing authorizations may be submitted either under a centralized, decentralized or mutual recognition procedure. The centralized procedure provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure includes selecting one “reference member state,” or RMS, and submitting to more than one member state at the same time. The RMS National Competent Authority conducts a detailed review and prepares an assessment report, to which concerned member states provide comment. The mutual recognition procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states post-initial approval. Within 90 days of receiving the applications and assessment report, each member state must decide whether to recognize the approval.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we obtain regulatory approval. In the United States and other markets, sales of any product candidates for which we receive regulatory approval for commercial sale will depend in part on the availability of coverage and reimbursement from third-party payors. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product. Third-party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the FDA-approved drug products for a particular indication.
64
Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. We may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of VERED and TWIN, in addition to the costs required to obtain the FDA approvals. For example, VERED and TWIN may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
In March 2010, the President of the United States signed the Affordable Care Act, one of the most significant healthcare reform measures in decades. The Affordable Care Act substantially changed the way healthcare is financed by both governmental and private insurers, and significantly impacted the pharmaceutical industry. The comprehensive USD$940 billion dollar overhaul ultimately extended coverage to approximately 31 million previously uninsured Americans. The Affordable Care Act contained a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse, which impacted existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program. Additionally, the Affordable Care Act: increased the minimum level of rebates payable by manufacturers of brand-name drugs from 15.1% to 23.1%; and imposed a non-deductible annual fee on pharmaceutical manufacturers or importers who sell “branded prescription drugs” to specific federal government programs. Since its enactment, there have been judicial and Congressional challenges to certain aspects of the Affordable Care Act. In 2017, the Tax Cuts and Jobs Act was enacted, which, among other things, removes penalties for not complying with the Affordable Care Act’s individual mandate to carry health insurance. It is uncertain the extent to which any such changes may impact our business or financial condition.
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. In August 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. Additionally, in January 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, further reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. Recently there has also been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several Congressional inquiries and proposed bills designed to, among other things, reform government program reimbursement methodologies. Individual states in the United States have also become increasingly active in passing legislation and implementing regulations designed to control pharmaceutical product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing. We expect that additional state and federal healthcare initiatives will be adopted in the future, any of which could impact the coverage and reimbursement for drugs, including our product candidates, if approved.
In the European Union, pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies or trials that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become intense. As a result, there are increasingly high barriers to entry for new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. Any country that has price controls or reimbursement limitations for drug products may not allow favorable reimbursement and pricing arrangements.
65
Healthcare Laws and Regulations
Our current and future business operations may be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and foreign jurisdictions in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security, price reporting and physician sunshine laws. Some of our pre-commercial activities are subject to some of these laws.
The federal Anti-Kickback Statute makes it illegal for any person or entity, including a prescription drug manufacturer or a party acting on its behalf to knowingly and willfully, directly or indirectly solicit, receive, offer, or pay any remuneration that is intended to induce the referral of business, including the purchase, order, lease of any good, facility, item or service for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, formulary managers, and beneficiaries on the other. Although there are a number of statutory exceptions and regulatory safe harbors protecting some common activities from prosecution, the exceptions and safe harbors are drawn narrowly. Practices that involve remuneration that may be alleged to be intended to induce prescribing, purchases or recommendations may be subject to scrutiny if they do not qualify for an exception or safe harbor. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered business, the Anti-Kickback Statute has been violated. In addition, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Violations of this law are punishable by up to five years in prison, and can also result in criminal fines, civil money penalties and exclusion from participation in federal healthcare programs. Moreover, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, for payment to, or approval by, federal programs, including Medicare and Medicaid, claims for items or services, including drugs, that are false or fraudulent or not provided as claimed. Persons and entities can be held liable under these laws if they are deemed to “cause” the submission of false or fraudulent claims by, for example, providing inaccurate billing or coding information to customers or promoting a product off-label. In addition, our future activities relating to the reporting of wholesaler or estimated retail prices for our product candidates, the reporting of prices used to calculate Medicaid rebate information and other information affecting federal, state and third-party reimbursement for our product candidates, and the sale and marketing of our product candidates, are subject to scrutiny under this law. Penalties for federal civil False Claims Act violations may include up to three times the actual damages sustained by the government, plus mandatory civil penalties for each separate false claim, the potential for exclusion from participation in federal healthcare programs, and, although the federal False Claims Act is a civil statute, False Claims Act violations may also implicate various federal criminal statutes.
HIPAA created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Also, many states have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs. Additionally, to the extent that any of our product candidates are sold in a foreign country, we may be subject to similar foreign laws.
66
HIPAA, as amended by HITECH, and their implementing regulations, including the final omnibus rule published on January 25, 2013, mandates, among other things, the adoption of uniform standards for the electronic exchange of information in common healthcare transactions, as well as standards relating to the privacy and security of individually identifiable health information, which require the adoption of administrative, physical and technical safeguards to protect such information. Among other things, HITECH makes HIPAA’s security standards directly applicable to business associates, defined as independent contractors or agents of covered entities that create, receive or obtain protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities and business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, certain state laws govern the privacy and security of health information in certain circumstances, some of which are more stringent than HIPAA and many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts. Failure to comply with these laws, where applicable, can result in the imposition of significant civil and/or criminal penalties.
The Affordable Care Act imposed, among other things, new annual reporting requirements for covered manufacturers for certain payments and other transfers of value provided to physicians and teaching hospitals, as well as certain ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately and completely the required information for all payments, transfers of value and ownership or investment interests may result in civil monetary penalties. Certain states also mandate implementation of compliance programs, impose restrictions on drug manufacturer marketing practices, require reporting of marketing expenditures and pricing information and/or require the tracking and reporting of gifts, compensation and other remuneration to physicians.
Because we intend to commercialize products that could be covered by a federal healthcare program and other governmental healthcare programs, we intend to develop a comprehensive compliance program that establishes internal controls to facilitate adherence to the rules and program requirements to which we will or may become subject. Although the development and implementation of compliance programs designed to establish internal controls and facilitate compliance can mitigate the risk of investigation, prosecution, and penalties assessed for violations of these laws, the risks cannot be entirely eliminated.
If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, contractual damages, reputational harm, diminished profits and future earnings, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and individual imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Our Challenges
We face challenges, risks and uncertainties in realizing our business objectives and executing our strategies, including:
• we are a growth-stage company with a history of losses, and we expect to incur significant expenses and continuing losses for the near-term;
• we have experienced growth and expect to invest in growth for the foreseeable future. If we fail to manage our growth effectively, our business, operating results and financial condition could be adversely affected;
• we currently face competition from a number of companies and expect to face significant competition in the future in our market;
• if we are unable to protect our intellectual property rights, our business, competitive position, financial condition and results of operations could be materially and adversely affected;
• non-compliance with requirements imposed by government patent agencies in jurisdictions where we have patent protection could reduce or eliminate our patent protection;
• intellectual property rights do not necessarily address all potential threats;
• we face risks related to health pandemics, including the ongoing COVID-19 pandemic, which could have a material adverse effect on our business and results of operations;
67
• we are expanding our operations internationally, which will expose us to additional tax, compliance, market and other risks;
• we will incur increased expenses and administrative burdens as an Australian public company treated as a public company in the United States, which could have an adverse effect on our business, financial condition and results of operations;
• we may be adversely affected by foreign currency fluctuations;
• any failure to comply with anticorruption and anti-money laundering laws, including the FCPA and similar laws associated with activities outside of the United States, could subject us to penalties and other adverse consequences;
• we could be adversely impacted if we fail to comply with U.S. and international import and export laws; and
• any failure to comply with laws relating to labor and employment could subject us to penalties and other adverse consequences.
Please see “Risk Factors” and other information included in this prospectus for a discussion of these and other risks and uncertainties that we face.
Employees
As of December 31, 2024, we had seven full-time employees, one part-time employee and ten consultants covering the following functions: sales, operations and marketing (6), finance and legal (5), manufacturing and R&D (6) and regulatory and intellectual property (1).
Our full and part-time employees and consultants are situated across Australia (12), the United States (4) and the United Kingdom (2).
We have entered into employment contracts with all of our full-time employees and consulting agreements with all of our part time staff and consultants. In addition to salaries and benefits, we have provided performance-based incentives for some of our full-time employees to create an incentive for them to remain as full-time employees.
Facilities
Our headquarters is located at Monash Innovation Labs, G. 60, 22 Alliance Lane, Clayton 3800, Victoria, Australia. On February 2, 2024, we and Monash University in Melbourne (“Monash”) entered into an agreement for the rental of laboratory facilities to support our research activities. The facility arrangement would also allow us to better engage with Monash staff, student and graduates for our verticals. Under the agreement, in consideration of AUD$10,644.75 per month, Monash shall provide 63 square meters of laboratory space and scientific equipment contained therein to conduct further research. The initial term began on February 5, 2024 until February 2, 2025, which was extended for a further term beginning from February 2, 2025.
Legal Proceedings
From time to time, we are involved in litigation or other legal proceedings incidental to our business. We are not currently a party to any litigation the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business, operating results, cash flows or financial condition.
68
DIRECTORS, SENIOR MANAGEMENT AND KEY EMPLOYEES
Set forth below is information concerning our directors and executive officers.
|
Name |
Age |
Position(s) |
||
|
Nathan J. Givoni |
41 |
Chief Executive Officer and Director (Board Member) |
||
|
Thuy-Linh Gigler |
45 |
Chief Financial Officer |
||
|
Dr. Paul M. Wynne |
62 |
Chief Scientific Officer |
||
|
Simon H. Szewach |
46 |
Director (Board Member) |
||
|
Jeffrey W. Olyniec |
50 |
Independent Director (Board Member) |
||
|
Hon. Philip A. Dalidakis |
49 |
Independent Director (Board Member) |
Management
Nathan J. Givoni
Nathan J. Givoni is one of our co-founders, our Chief Executive Officer and a Director of the Company since inception. Mr. Givoni is a health professional with over 15 years of experience in the health and medical fields. His responsibilities to the Company include oversight of (i) the day-to-day operation of our business, (ii) day-to-day science and formulations of new and existing product, (iii) manufacturing and supply chain of our business, (iv) all intellectual property matters relating to our business and (v) the suppliers to our business. He is the founder and Managing Director of Lifestyle Breakthrough Pty Ltd, a medical and allied health consulting service with locations across Australia, from July 2011 to 2023. Mr. Givoni is also the founder of the Metabolic Health Foundation, founded in Australia in March 2022 to present.
Mr. Givoni received a Bachelor of Science in Physiology & Psychology in 2006, a Bachelor of Science in Physiology (First Class Honors) in 2007 and a Bachelor of Nutrition and Dietetics in 2009 respectively from Monash University. He worked as an adjunct lecturer at Monash University from 2014 to 2017, publishing multiple papers post his undergraduate degree. He has trained and worked as both a dietitian and exercise physiologist, bringing clinical knowledge to our business. We believe that Mr. Givoni’s extensive background as a health professional and his academic knowledge related to nutritional sciences qualifies him to serve on our Board.
Thuy-Linh Gigler
Thuy-Linh Gigler has been the Chief Financial Officer of the Company since June 2025. Mrs. Gigler is employed as Head of CFO and Treasury Services at Vistra, a global professional services firm, since September 2024 and was Head of Finance at Vistra from October 2022 to August 2024. At Vistra, Mrs. Gigler provides CFO and Treasury services to a portfolio of ASX listed and unlisted companies in the technology, medical services and mineral exploration sectors. Prior to this role, Mrs. Gigler was Financial Controller at Tata Consumer Products Australia Pty Ltd from June 2010 to May 2022 and Finance Manager at Peter Rowland Catering Pty Ltd from May 2006 to June 2010.
Mrs. Gilger qualified as a Certified Public Accountant in 2007 and received a Bachelor of Commerce from the University of Melbourne in 2003.
Dr. Paul M. Wynne
Dr. Paul Wynne has been our Chief Scientific Officer since November 2024. He has over 35 years of experience in the disciplines of analytical chemistry, the design and manufacture of advanced materials, drug metabolism, pharmaceutical formulation, drug delivery and forensic toxicology. Prior to joining Gelteq, he was the Manager of the Medicines Manufacturing Innovation Centre at Monash University in Melbourne, which works to strengthen the pharmaceutical and allied manufacturing sector in Australia for domestic and international clients from November 2016 to October 2024. He is the author of many reviewed papers, book chapters, patents, lectures, presentations and industry technical articles.
Dr. Wynne received a Bachelor of Applied Science in Applied Chemistry in 1984, Master of Applied Science in Organic Photochemistry in 1987 and a Doctor of Philosophy in Chemistry and Toxicology in 2001 from RMIT University.
69
Directors
The following noteworthy experience, qualifications, attributes and skills for our directors, together with the biographical information for each independent director described below, led to our conclusion that such persons should serve as our directors in light of our business and structure:
Simon H. Szewach
Simon H. Szewach is one of our co-founders and served as our Executive Chairman of the Board of Directors of the Company from August 2021 until March 2025. He has served as a Director on our Board of Directors since April 1, 2025. He has extensive experience in commercial sales and marketing of new products trends in the finance, technology and sport sectors. His prior work experience in sales, marketing and technology includes serving as a managing partner of The Legats Group, a Melbourne-based company that invests in leading-edge start-ups with strong competitive advantages through innovative technologies and intellectual property, from November 2016 to present. Prior to that, Mr. Szewach served as the co-founder and managing director of nTouch Pty Ltd, a proximity-based marketing platform, from 2013 to 2015. In 2015, YPB group Ltd (ASX:YPB), a brand protection company, acquired nTouch Pty Ltd, and Mr. Szewach then served as President of Consumer Engagement at YPB Group Ltd from 2015 to 2017. He was the co-founder and chief executive officer of StartHere.com.au, an incentive-based shopping platform, from 2012 to 2015. Mr. Szewach is also the co-founder and a member of the board of directors of the Sports Diplomacy Alliance, founded in September 2021, and is also on the board of directors of Global Reviews Holding Pty Ltd, from June 2012 to present
Mr. Szewach received both his Bachelor of Business in Banking & Finance and a Bachelor of Arts in Asian Studies (Korean) respectively from Monash University in 2003. We believe that Mr. Szewach’s extensive knowledge of our Company as founder and his experience in executive roles across multiple start-ups qualifies him to serve on our Board.
Jeffrey W. Olyniec
Jeffrey W. Olyniec has been an independent director on our board of directors since August 2021. He is currently a General Partner at Moneta Ventures since January 2024, an early-stage venture capital firm based in Northern California. He has over twenty-five years of living and working experience in the People’s Republic of China, where he formed and has led multiple companies. He was the co-founder and Chief Executive Officer of New Vision Display Inc from October 2012 until December 2023, a manufacturer of custom display and touch solutions (SZSE: 300120). Additionally, he is the co-founder and a member of the board of directors of PacificPine Sports Limited from August 2012, a China-based sports academy group; the co-founder and Executive Chairman of GP87, Inc. from February 2014, a manufacturer of snowboards, skis, surfboards and foil boards; the co-founder and Deputy Chairman of Nine Rivers Distillery Ltd. from December 2018, a distillery in Fujian Province, China; and the Executive Chair of ReviverMx, Inc. from December 2019, a digital license plate company.
Mr. Olyniec received a Bachelor of Business Administration from Mississippi State University in 1998 and speaks fluent Mandarin Chinese. We believe that Mr. Olyniec’s background as a founder of various start-ups and his current management positions in small and medium sized enterprises qualifies him to serve on our Board.
Hon. Philip A. Dalidakis
The Hon. Philip Dalidakis has been an independent director on our board of directors since April 2022. He is a political, business and industry leader in Australia with experience in federal and state government and had held executive corporate roles at businesses in Australia. He is currently the managing partner of Orizontas from July 2020, a boutique corporate advisory consultancy based in Melbourne, Australia that solves business challenges through strategic advice and deep expertise in political, market, reputational and climate risk.
He served as the Executive General Manager, Corporate Services at Australia Post, formerly the Australian Postal Corporation, the government business enterprise that provides postal services in Australia, from July 2019 to April 2020, where he was responsible for communications, corporate secretarial, legal, regulatory affairs and strategy functions. Prior to this, he served as the Victorian Minister for Innovation and the Digital Economy, Trade and Investment and Small Business and as a member of the Parliament of Victoria, which is the bicameral
70
legislature of the Australian state of Victoria, from December 2014 to June 2019. As the Innovation Minister, he positioned the Australian state of Victoria as a leading biotech, innovation & technology hub across the Asia Pacific, where he executed a strategy that attracted APAC/ANZ head offices of global tech companies such as GoPro Inc. (NASDAQ: GPRO), Hire Technologies Inc. (OTCMKTS: HIRRF), Slack Technologies, Block, Inc. (formerly named Square, Inc. and d/b/a Square) (NYSE: SQ), Stripe, Inc. (d/b/a Stripe) and Zendesk Inc. (NYSE: ZEN) into Melbourne.
The Hon. Philip Dalidakis has been appointed by the Australian government, since January 2025, to represent the government on the Asia Pacific Economic Cooperation (APEC) Business Advisory Council for a term of three years. He also currently serves as a director on the board of directors of various institutions including as Chairman of VOICsa, a not for profit charity for victims of child sex abuse, the Washington D.C. based Center for Asia Pacific Strategy from April 2020. He previously sat on the board of directors of GrowthOps Ltd (ASX: TGO), an Australian-based growth experience company that drives competitive growth for its corporate clients, chairing its Audit and Risk Committee from October 2019 to November 2021.
Mr. Dalidakis received both a Bachelor of Business in Management and a Bachelor of Arts in Politics and Thai Language in 2000 from Monash University and a Masters of Commerce from the University of New South Wales in 2003. We believe that the Hon. Dalidakis’ background in business advisory, public service and experience as director in listed companies qualifies him to serve on our Board.
Family Relationships
None of our directors or executive officers has a family relationship as defined in Item 401 of Regulation S-K.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has, during the past 10 years, been involved in any legal proceedings described in subparagraph (f) of Item 401 of Regulation S-K.
Board of Directors
Our board of directors consists of five directors, two of whom are “independent” within the meaning of Section 5605(a)(2) of the NASDAQ Listing Rules and meet the criteria for independence as set forth in Rule 10A-3 of the Exchange Act. As of the date of this prospectus, we had one executive director (Nathan J. Givoni), two non-independent directors (Nathan J. Givoni and Simon H. Szewach) and two independent directors (Jeffrey W. Olyniec and Hon Phillip Dalidakis).
Terms of Directors and Executive Officers
Our officers are appointed by and serve at the discretion of our board of directors. Our directors are not subject to a set term of office and hold office until the next general meeting called for the appointment of directors and until their successor is duly appointed or such time as they die, resign or are removed from office by a shareholders’ ordinary resolution. The office of a director will be vacated automatically if, among other things, the director resigns in writing, becomes bankrupt or makes any arrangement or composition with his or her creditors generally or is found to be or becomes of unsound mind.
Qualification
There is currently no shareholding qualification for directors, although a shareholding qualification for directors may be fixed in the future by our shareholders by ordinary resolution.
Committees of the Board of Directors
We have established three Committees of our board of directors: an Audit Committee, a Compensation Committee, and a Nominating and Corporate Governance Committee. We have adopted a formal charter for each of the Audit and Risk Management Committee Charter, Compensation and Nominating and Governance committees. We have
71
determined that Mr. Olyniec and Mr. Dalidakis satisfy the “independence” requirements of Section 5605(a)(2) of the Nasdaq Listing Rules and Rule 10A-3 under the Securities Exchange Act. Each Committee’s members and functions are described below.
Members will serve on these committees until their resignation or until otherwise determined by our board of directors.
Audit Committee. Each of our Audit Committee members satisfies the “independence” requirements of the NASDAQ listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. We have determined that Mr. Dalidakis possesses the accounting or related financial management experience that qualifies him as an “audit committee financial expert” as defined by the rules and regulations of the SEC. The Audit Committee oversees our accounting and financial reporting processes and the audits of the financial statements of our company. The Audit Committee is responsible for, among other things:
• appointing the independent auditors and pre-approving all auditing and non-auditing services permitted to be performed by the independent auditors;
• reviewing with the independent auditors any audit problems or difficulties and management’s response;
• discussing the annual audited financial statements with management and the independent auditors;
• reviewing the adequacy and effectiveness of our accounting and internal control policies and procedures and any steps taken to monitor and control major financial risk exposures;
• reviewing and approving all proposed related party transactions;
• meeting separately and periodically with management and the independent auditors;
• monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance; and
• review the Company’s risk management framework including in relation to economic, environmental, and social sustainability risk at least annually.
The members of the Audit Committee are Mr. Dalidakis and Mr. Olyniec. Mr. Dalidakis is the chairperson of the Audit Committee. We are drawing upon Mr. Dalidakis’ prior experience as a director on the board of directors of various institutions including as the chair of the audit and risk committee of another Australian-based company in naming him as the chairperson of the Audit Committee.
Compensation Committee. All of our Compensation Committee members satisfy the “independence” requirements of the NASDAQ listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. The Compensation Committee assist the board in reviewing and approving the compensation structure, including all forms of compensation, relating to our directors and executive officers. Our chief executive officer may not be present at any Committee meeting during which his compensation is deliberated. The Compensation Committee is responsible for, among other things:
• reviewing and approving the total compensation package for our most senior executive officers;
• approving and overseeing the total compensation package for our executives other than the most senior executive officers;
• reviewing and recommending to the board with respect to the compensation of our directors;
• reviewing periodically and approving any long-term incentive compensation or equity plans;
• selecting compensation consultants, legal counsel or other advisors after taking into consideration all factors relevant to that person’s independence from management; and
• reviewing programs or similar arrangements, annual bonuses, employee pension and welfare benefit plans.
The members of the Compensation Committee are Mr. Dalidakis and Mr. Olyniec. Mr. Olyniec is the chairperson of the Compensation Committee.
72
Nominating and Corporate Governance Committee. A majority of our Nominating and Corporate Governance Committee members satisfy the “independence” requirements of the NASDAQ listing rules and meet the independence standards under Rule 10A-3 under the Exchange Act. The Nominating and Corporate Governance Committee assists our board of directors in selecting individuals qualified to become our directors and in determining the composition of the board and its Committees. The Nominating and Corporate Governance Committee is responsible for, among other things:
• identifying and recommending nominees for election or re-election to our board of directors or for appointment to fill any vacancy;
• reviewing annually with our board of directors its current composition in light of the characteristics of independence, age, skills, experience and availability of service to us;
• identifying and recommending to our board of directors to serve as members of Committees;
• advising the board periodically with respect to significant developments in the law and practice of corporate governance as well as our compliance with applicable laws and regulations, and making recommendations to our board of directors on all matters of corporate governance and on any corrective action to be taken; and
• monitoring compliance with our code of business conduct and ethics, including reviewing the adequacy and effectiveness of our procedures to ensure proper compliance.
The members of the Nominating and Corporate Governance Committee are Mr. Dalidakis and Mr. Olyniec. Mr. Dalidakis and Mr. Olyniec share chairperson duties, with Mr. Dalidakis chairing Corporate Governance matters and Mr. Olyniec chairing Nominating matters.
Qualification
There is currently no shareholding qualification for our directors, although a shareholding qualification for directors may be fixed in the future by our shareholders by ordinary resolution.
Code of Business Conduct and Ethics
Our Board has adopted a Code of Business Conduct and Ethics which codifies the business and ethical principles that govern all aspects of our business and complies with the standards expected of NASDAQ listed companies. You may be able to review these documents by accessing our public filings at the SEC’s website at www.sec.gov.
Duties of Directors
Under Australian law, our directors have a duty to act honestly, in good faith and in the best interests of all shareholders. Our directors also have a duty to exercise the care, diligence and skills that a reasonably prudent person would exercise in comparable circumstances. In fulfilling their fiduciary duty to the shareholders of the Company, our directors must ensure compliance with our Constitution on and after the closing of our initial public offering. Our shareholders may have the right to seek damages from either the Company, the directors personally, or both, if a duty owed by our directors is breached.
The functions and powers of our board of directors include, among others:
• appointing officers and determining the term of office of the officers;
• exercising the borrowing powers of the company and mortgaging the property of the Company;
• executing checks, promissory notes and other negotiable instruments on behalf of the Company;
• maintaining or registering a register of mortgages, charges or other encumbrances of the company; and
• adopting any scheme or plan in the best interests of the Company designed to provide retiring or superannuation benefits for both present and future non-executive directors;
73
• delegating any of their powers to a committee consisting of such of their number as they may determine; and
• appointing any person to be attorney of the Company.
Non-Employee Director Compensation
We have not historically had a formal compensation policy with respect to service on our board of directors, but we have reimbursed our non-employee directors for out-of-pocket direct expenses incurred in connection with attending meetings on our behalf.
We expect our board to approve a non-employee director compensation policy intended to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors’ interests with those of our shareholders. Under this policy, we will pay each of our non-employee directors a cash retainer for service on the board of directors and for service on each committee on which the director is a member.
The chairperson of each committee will receive a higher retainer for such service. These retainers are payable, provided we have the requisite cash available, in arrears in four equal quarterly installments on the last day of each quarter, provided that the amount of such payment will be prorated for any portion of such quarter that the director is not serving on our board of directors or the applicable committee. The retainers to be paid to non-employee directors for service on the board of directors and for service on each committee of the board of directors on which the director is a member are as follows:
|
Position |
Annual |
Chairperson |
||||||
|
Board of Directors |
USD$ |
25,000 |
USD$ |
5,000 |
||||
|
Audit Committee |
USD$ |
5,000 |
USD$ |
5,000 |
||||
|
Compensation Committee |
USD$ |
5,000 |
USD$ |
5,000 |
||||
|
Nominating and Corporate Governance Committee |
USD$ |
5,000 |
USD$ |
5,000 |
||||
In addition, non-employee directors will be eligible to participate in the proposed Incentive Plan and may be granted share options and/or restricted shares under the proposed Incentive Plan from time to time.
74
EXECUTIVE COMPENSATION
Executive Compensation
As described below, we plan to adopt an incentive plan in the fiscal year ending June 30, 2026. Our proposed incentive plan will include our named executive officers. As of the date of this prospectus, we do not have any equity-based incentive awards.
Agreements with Named Executive Officers
Nathan J. Givoni — Co-Founder, Chief Executive Officer and Director.
The Company entered into an employment agreement with Mr. Givoni in April 2021. Mr. Givoni serves as the Company’s Chief Executive Officer and Director and receives an annual compensation of USD$300,000 plus an agreed level of STI and ESOP coverage should the Company decide to implement such a program. Mr. Givoni oversees the daily business operations together with product development, scientific research, intellectual property and manufacturing. The employment agreement stipulates that Mr. Givoni must give six months written notice of his intent to resign, allowing the Company to find a suitable replacement. In October, 2024, the Compensation Committee and Mr. Givoni agreed to lower the total renumeration payable by the Company to Mr. Givoni to USD$167,000 per year to preserve the Company’s capital and to improve the Company’s fiscal management.
Thuy — Linh Gigler — Chief Financial Officer
The Company entered into an agreement with Vistra (Australia) Pty Ltd (“Vistra”), under which Mrs. Thuy-Linh Gigler, serves as our Chief Financial Officer, on February 2024. Mrs. Gigler has been selected to join the Company as CFO in June 2025. The CFO Services Agreement has an indefinite term and shall continue thereafter until terminated by our Company with sixty days written notice at any time or by Vistra by sixty days written notice at any time. Under the CFO Services Agreement, the Company has agreed to pay a monthly renumeration of at least USD$2,304 (AUD$3,200) to Vistra for Mrs. Gigler’s services. Mrs. Gigler oversees the Company’s financial strategy.
Dr. Paul M. Wynne — Chief Scientific Officer
The Company entered into an employment agreement with Dr. Wynne in October 2024. Dr. Wynne serves as the Company’s Chief Scientific Officer and Director and receives an annual compensation of USD$136,500 plus an agreed level of STI and ESOP coverage should the Company decide to implement such a program. Dr. Wynne overseas our research & development and scientific development team. Dr. Wynne is subject to customary non-solicitation, non-compete and exclusivity clauses in accordance with his employment agreement. The employment agreement stipulates that Dr. Wynne must give four weeks written notice of his intent to resign, allowing the Company to find a suitable replacement.
Engagement of Executives
Equity Incentive Plan
We expect our board to approve a non-employee director compensation policy in 2026 intended to provide a total compensation package that enables us to attract and retain qualified and experienced individuals to serve as directors and to align our directors’ interests with those of our shareholders. Under this policy, we will pay each of our non-employee directors a cash retainer for service on the board of directors and for service on each committee on which the director is a member.
Shares Subject to the equity incentive plan
We expect up to 10% of our Ordinary Shares to be available for issuance under the equity incentive plan. If an award granted under the equity incentive plan is forfeited, canceled, settled, or otherwise terminated without a distribution of Ordinary Shares, the Ordinary Shares underlying that award will again become available for issuance under the equity incentive plan. If Ordinary Shares delivered under the Plan are tendered or withheld to pay the exercise price of a share option or to satisfy withholding taxes, those Ordinary Shares will also again become available for issuance under the equity incentive plan.
75
PRINCIPAL SHAREHOLDERS
Except as specifically noted, the following table sets forth information with respect to the beneficial ownership of our Ordinary Shares as of the date of this prospectus by:
• each of our directors and executive officers; and
• each person known to us to beneficially own more than 5% of our Ordinary Shares on an as-converted basis.
The calculations in the table below are based on 10,028,025 Ordinary Shares outstanding as of the date of this prospectus.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, we have included shares that the person has the right to acquire within 60 days, including through the exercise of any option, warrant or other right or the conversion of any other security. These shares, however, are not included in the computation of the percentage ownership of any other person.
|
Total |
% of |
|||||
|
Directors and Executive Officers: |
|
|
||||
|
Simon H. Szewach |
338,197 |
(1) |
3.37 |
% |
||
|
Nathan J. Givoni |
657,087 |
(2) |
6.55 |
% |
||
|
Jeffrey W. Olyniec |
164,809 |
|
1.64 |
% |
||
|
Thuy – Linh Gigler |
— |
|
— |
|
||
|
|
|
|||||
|
Hon. Philip A. Dalidakis |
— |
|
— |
|
||
|
Total of all directors and executive officers (5 persons) |
1,160,093 |
|
11.57 |
% |
||
|
5% Shareholders: |
|
|
||||
|
Crestmont Pty Ltd ATF Crestmont Investments Trust(3) |
642,323 |
|
6.41 |
% |
||
|
David Golik(4) |
1,012,288 |
|
10.09 |
% |
||
|
Jeffrey Markoff(5) |
2,440,734 |
|
24.34 |
% |
||
____________
(1) Consists of (i) 153,300 Ordinary Shares held by Legats Pty Ltd ATF The Simon Szewach Family Trust (“Legats”), and (ii) 184,897 Ordinary Shares held by Domalina Pty Ltd ATF Domalina Investments Trust (“Domalina”). Legats is a privately owned discretionary trust and Domalina is a unit trust. The Ordinary Shares of Legats and Domalina are beneficially held by Simon H. Szewach. Does not include Ordinary Shares held by Chaplin Investments Pty Ltd as trustee for Chaplin Investments Trust (“Chaplin”), a privately owned discretionary trust. Because Simon H. Szewach, as one of the potential beneficiaries of Chaplin, does not have investment and voting power of the Ordinary Shares, he is not deemed to be a beneficial owner of the Ordinary Shares held by Chaplin. Mr. Szewach had been our Executive Chairman of the Board of Directors of the Company from August 2021 until his resignation on March 31, 2025. He has served as a Director on our Board of Directors since April 1, 2025.
(2) Consists of (i) 487,988 Ordinary Shares held by Lorch Investments Pty Ltd ATF Lorch Investments Trust (“Lorch”), (ii) 76,650 Ordinary Shares held by Givoni Investments Pty Ltd ATF Givoni Investments Family Trust (“Givoni Investments Trust”) and (iii) 92,449 Ordinary Shares held by Domalina Pty Ltd ATF Domalina Investments Trust (“Domalina”). Givoni Investments Trust and Lorch are privately owned discretionary trusts and Domalina is a unit trust. The Ordinary Shares of Lorch, Givoni Investments Trust and Domalina are beneficially held by Nathan J. Givoni.
(3) Crestmont Pty Ltd ATF Crestmont Investments Trust is a privately owned discretionary trust beneficially held by Mark Saltzman.
(4) Consists of (i) 975,975 Ordinary Shares held by Chaplin Investments Pty Ltd (“Chaplin”) and (ii) 36,313 Ordinary Shares held by Caddarly Pty Ltd ATF Golik Family Trust No 2 (“Caddarly”). Chaplin and Caddarly are privately owned discretionary trusts. The Ordinary Shares of Chaplin and Caddarly are beneficially held by David Golik.
(5) Consists of (i) 1,748,992 Ordinary Shares held by ACK Pty Ltd ATF Markoff Superannuation Fund No.2 (“ACK”) and (ii) 691,742 Ordinary Shares held by FFOKRAM Pty Ltd ATF FFOKRAM Trust (“FFOKRAM”). ACK is a privately owned superannuation/pension fund that is beneficially held by Mr. Jeffrey Markoff and Ms. Yumi Markoff. FFOKRAM is a privately owned discretionary trust, beneficially held by Mr. Jeffrey Markoff.
76
RELATED PARTY TRANSACTIONS
Shareholder Loan Agreements
On January 20, 2022, we entered into separate Loan Agreements, among others, with B&M Givoni Pty Ltd ATF B&M Givoni Superannuation Fund (the “B&M Givoni Superannuation Fund”, and the loan entered with the foregoing fund, the “B&M Givoni Loan”) and our director Jeffrey W. Olyniec (the “Olyniec Loan”) for the provision of loans. The principal of the B&M Givoni Loan is $350,000, comprising debt of AUD$262,570 and an amount of Ordinary Shares equivalent to AUD$87,430 (approximately USD$170,671 and USD$56,830) and the principal of the Olyniec Loan is AUD$143,445, comprising debt of AUD$106,767 and an amount of Ordinary Shares equivalent to AUD$36,678 (approximately USD$69,399 and USD$23,841). Both the B&M Givoni Loan and the Olyniec Loan has an interest rate of 12% per annum maturing on July 15, 2023 to fund the expenses for the proposed listing and for working capital purposes. As part of this loan agreement, we agreed to issue AUD$1.00 of Ordinary Shares to the B&M Givoni Superannuation Fund and Jeffrey W. Olyniec for every AUD$4.00 of principal loaned to us. The Ordinary Shares were issued within 90 days of the loan being advanced. The B&M Givoni Superannuation Fund is our Chief Executive Officer and Director Nathan J. Givoni’s parents’ Superannuation fund or pension fund, with Nathan J Givoni having no ownership, title or beneficial interests in this entity. On January 3, 2023, both the B&M Givoni Loan and the Olyniec Loan were extended for an additional 12 months at an interest rate of 12% per annum maturing on July 15, 2024. Such extensions constitute a substantial modification per IFRS 9, and therefore the original liability is derecognized on modification date, and the new liability for the extended loans is recognized at fair value, discounted using an appropriate discount rate. As of June 30, 2023, the outstanding amount payable for both the B&M Givoni Loan and the Olyniec Loan is approximately AUD$483,601 (approximately USD$314,341). During October 2023, both loan holders agreed to further extend the loans with a new maturity date of December 31, 2024. In October 2024, both loan holders as described herein agreed to further extend the maturity date of their loan to December 31, 2025.
Provision of Services by Asiana Trading Corporation Limited
On July 1, 2021, we entered into a Consulting Services Agreement (the “Consulting Agreement”) with Asiana. Asiana introduces new products on behalf of their clients in China, including local sales marketing efforts, legal and compliance support, logistics services, and local supplier introductions. During the term of the Consulting Agreement, Asiana had provided management services to the Company to facilitate the Company’s services undertaken in China, including legal expenses, product samples and pre-paid expenses.
The Company paid Asiana AUD$177,065.09 (approximately USD$122,751.26) under the Consulting Agreement to reimburse certain operating costs of Asiana, which had five employees. While Mr. Olyniec was the sole shareholder of Asiana, from October 2020 until December of 2021 and one its two directors, he received none of the amounts paid to Asiana under the Consulting Agreement. As such, Mr. Olyniec was not reimbursed from the funds earned under the Consulting Agreement and did not perform the services stipulated in the Consulting Agreement. On December 25, 2021, Mr. Olyniec resigned as a director and is no longer is a director of Asiana.
During the year ended June 30, 2024, Asiana provided management and legal services to the Company of AUD$9,125 (USD$6,114) as compared to the year ended June 30, 2023 in the amount of AUD$171,530 (USD$123,502). for the six month period ended December 31, 2024, the Company paid Asiana in the amount of AUD $261,916 (USD$175,000) compared to the six month period ended December 31, 2023, AUD $18,900 (USD$12,500).
77
SELLING SHAREHOLDER
This prospectus relates to the possible resale by the selling shareholder, Lincoln Park, of Ordinary Shares that may be issued to Lincoln Park pursuant to the Purchase Agreement. We are filing the registration statement of which this prospectus forms a part pursuant to the provisions of the Registration Rights Agreement, which we entered into with Lincoln Park on March 13, 2025, concurrently with our execution of the Purchase Agreement, in which we agreed to provide certain registration rights with respect to sales by Lincoln Park of the shares that may be issued to Lincoln Park under the Purchase Agreement.
Lincoln Park, as the selling shareholder, may from time to time offer and sell pursuant to this prospectus any or all of the Ordinary Shares that we may sell to Lincoln Park under the Purchase Agreement. The selling shareholder may sell some, all, or none of its shares. We do not know how long the selling shareholder will hold the shares before selling them, and we currently have no agreements, arrangements, or understandings with the selling shareholder regarding the sale of any of the shares.
The following table provides, as of June 27, 2025, information regarding the selling shareholder and the Ordinary Shares that it may offer and sell from time to time under this prospectus. The percentage of ownership in the table below is based on 10,028,025 Ordinary Shares outstanding on the date prior to the filing of this prospectus, including the 175,000 Commitment Shares we have already issued to Lincoln Park pursuant to the Purchase Agreement. The table is prepared based on information supplied to us by the selling shareholder, and reflects its holdings as of June 27, 2025. Neither Lincoln Park nor any of its affiliates has held a position or office, or had any other material relationship, with us or any of our predecessors or affiliates. Beneficial ownership is determined in accordance with Section 13(d) of the Exchange Act and Rule 13d-3 thereunder.
|
Selling Shareholder |
Ordinary |
Percentage of |
Ordinary |
Percentage of |
||||||||
|
Lincoln Park Capital Fund, LLC(1) |
175,000 |
(2) |
1.74 |
% |
4,000,000 |
(3) |
0 |
%(4) |
||||
____________
(1) Josh Scheinfeld and Jonathan Cope, the Managing Members of Lincoln Park Capital, LLC, are deemed to be beneficial owners of all of the Ordinary Shares owned by Lincoln Park Capital Fund, LLC. Messrs. Cope and Scheinfeld have shared voting and investment power over the shares being offered under the prospectus in connection with the transactions contemplated under the Purchase Agreement. Lincoln Park Capital, LLC is not a licensed broker dealer or an affiliate of a licensed broker dealer.
(2) Represents the 175,000 Commitment Shares issued to Lincoln Park on March 13, 2025 as a fee for its commitment to purchase shares under the Purchase Agreement, all of which are covered by the registration statement that includes this prospectus. We have excluded from the number of shares beneficially owned by Lincoln Park prior to the offering all of the Ordinary Shares that Lincoln Park may be required to purchase on or after the date of this prospectus pursuant to the Purchase Agreement, because the issuance of such shares is solely at our discretion and is subject to certain conditions, the satisfaction of all of which are outside of Lincoln Park’s control, including the registration statement of which this prospectus is a part becoming and remaining effective. Furthermore, under the terms of the Purchase Agreement, issuances and sales of our Ordinary Shares to Lincoln Park are subject to certain limitations on the amounts we may sell to Lincoln Park at any time, including the Beneficial Ownership Limitation. See the description under the heading “Lincoln Park Transaction” for more information about the Purchase Agreement.
(3) Although the Purchase Agreement provides that we may sell up to $12,000,000 of Ordinary Shares to Lincoln Park, we are only registering 4,000,000 Ordinary Shares for resale under this prospectus, including the 175,000 Commitment Shares. Therefore, only 3,825,000 of such shares represent shares that we may issue and sell to Lincoln Park for cash consideration in purchases under the Purchase Agreement from time to time, at our sole discretion, during the 24-month period commencing on the Commencement Date. Depending on the price per share at which we sell our Ordinary Shares to Lincoln Park pursuant to the Purchase Agreement, we may need to sell to Lincoln Park under the Purchase Agreement more Ordinary Shares than are offered under this prospectus in order to receive aggregate gross proceeds equal to the full $12.0 million available to us under the Purchase Agreement. If we choose to do so, we must first register for resale under the Securities Act such additional shares. The number of shares ultimately offered for resale by Lincoln Park is dependent upon the number of shares we sell to Lincoln Park under the Purchase Agreement. Additionally, in no event may we issue or sell to Lincoln Park under the Purchase Agreement Ordinary Shares in excess of 1,881,328 shares, which is equal 19.99% of our Ordinary Shares outstanding immediately prior to the execution of the Purchase Agreement (the “Exchange Cap”) unless (i) we obtain shareholder approval to issue our Ordinary Shares in excess of the Exchange Cap or (ii) the average price of all Ordinary
78
Shares issued to Lincoln Park under the Purchase Agreement equals or exceeds $1.29 per share (which represents the lower of (A) the official closing price of the Ordinary Shares on Nasdaq immediately preceding the signing of the Purchase Agreement and (B) the average official closing price of the Ordinary Shares on Nasdaq for the five consecutive trading days ending on the trading day immediately preceding the date of the Purchase Agreement), such that the transactions contemplated by the Purchase Agreement are exempt from the Exchange Cap limitation under applicable Nasdaq rules. In any event, the Purchase Agreement specifically provides that we may not issue or sell any Ordinary Shares under the Purchase Agreement if such issuance or sale would breach any applicable rules or regulations of Nasdaq or the Corporations Act 2001 (Commonwealth of Australia).
(4) Assumes the sale of all Ordinary Shares registered pursuant to this prospectus, although the selling shareholder is under no obligation to sell any Ordinary Shares at this time.
79
PLAN OF DISTRIBUTION
The Ordinary Shares offered by this prospectus are being offered by Lincoln Park. The Ordinary Shares may be sold or distributed from time to time by Lincoln Park directly to one or more purchasers or through brokers, dealers, or underwriters who may act solely as agents at market prices prevailing at the time of sale, at prices related to the prevailing market prices, at negotiated prices, or at fixed prices, which may be changed. The sale of the Ordinary Shares offered by this prospectus could be effected in one or more of the following methods:
• ordinary brokers’ transactions;
• transactions involving cross or block trades;
• through brokers, dealers, or underwriters who may act solely as agents;
• “at the market” into an existing market for the Ordinary Shares;
• in other ways not involving market makers or established business markets, including direct sales to purchasers or sales effected through agents;
• in privately negotiated transactions; or
• any combination of the foregoing.
In order to comply with the securities laws of certain states, if applicable, the Ordinary Shares may be sold only through registered or licensed brokers or dealers. In addition, in certain states, the Ordinary Shares may not be sold unless they have been registered or qualified for sale in the state or an exemption from the state’s registration or qualification requirement is available and complied with.
Lincoln Park is an “underwriter” within the meaning of Section 2(a)(11) of the Securities Act.
Lincoln Park has informed us that it intends to use an unaffiliated broker-dealer to effectuate all sales, if any, of the Ordinary Shares that it has acquired and may in the future acquire from us pursuant to the Purchase Agreement. Such sales will be made at prices and at terms then prevailing or at prices related to the then current market price. Each such unaffiliated broker-dealer will be an underwriter within the meaning of Section 2(a)(11) of the Securities Act. Lincoln Park has informed us that each such broker-dealer will receive commissions from Lincoln Park that will not exceed customary brokerage commissions.
Brokers, dealers, underwriters or agents participating in the distribution of the Ordinary Shares offered by this prospectus may receive compensation in the form of commissions, discounts, or concessions from Lincoln Park, for whom the broker-dealers may act as agent. The compensation paid to any such particular broker-dealer may be less than or in excess of customary commissions. Neither we nor Lincoln Park can presently estimate the amount of compensation that any agent will receive for any purchases of Ordinary Shares sold by Lincoln Park.
We know of no existing arrangements between Lincoln Park or any other shareholder, broker, dealer, underwriter or agent relating to the sale or distribution of the Ordinary Shares offered by this prospectus.
We may from time to time file with the SEC one or more supplements to this prospectus or amendments to the registration statement of which this prospectus forms a part to amend, supplement or update information contained in this prospectus, including, if and when required under the Securities Act, to disclose certain information relating to a particular sale of Ordinary Shares offered by this prospectus by Lincoln Park, including the names of any brokers, dealers, underwriters or agents participating in the distribution of such Ordinary Shares to Lincoln Park, any compensation paid by Lincoln Park to any such brokers, dealers, underwriters or agents, and any other required information.
We will pay the expenses incident to the registration under the Securities Act of the offer and sale of the Ordinary Shares covered by this prospectus by Lincoln Park. We have agreed to indemnify Lincoln Park and certain other persons against certain liabilities in connection with the offering of Ordinary Shares offered hereby, including liabilities arising under the Securities Act or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities. Lincoln Park has agreed to indemnify us against liabilities under the Securities Act that may arise from certain written information furnished to us by Lincoln Park specifically for use in this prospectus or, if such indemnity is unavailable, to contribute amounts required to be paid in respect of such liabilities.
80
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers, and controlling persons, we have been advised that in the opinion of the SEC this indemnification is against public policy as expressed in the Securities Act and is therefore, unenforceable.
Lincoln Park has represented to us that at no time prior to the Purchase Agreement has Lincoln Park or its agents, representatives or affiliates engaged in or effected, in any manner whatsoever, directly or indirectly, any short sale (as such term is defined in Rule 200 of Regulation SHO of the Exchange Act) of our Ordinary Shares or any hedging transaction, which establishes a net short position with respect to our Ordinary Shares. Lincoln Park agreed that during the term of the Purchase Agreement, it, its agents, representatives or affiliates will not enter into or effect, directly or indirectly, any of the foregoing transactions.
We have advised Lincoln Park that it is required to comply with Regulation M promulgated under the Exchange Act. With certain exceptions, Regulation M precludes Lincoln Park, any affiliated purchasers, and any broker-dealer or other person who participates in the distribution from bidding for or purchasing, or attempting to induce any person to bid for or purchase any security which is the subject of the distribution until the entire distribution is complete. Regulation M also prohibits any bids or purchases made in order to stabilize the price of a security in connection with the distribution of that security. All of the foregoing may affect the marketability of the securities offered by this prospectus.
This offering will terminate on the date that all Ordinary Shares offered by this prospectus have been sold by Lincoln Park.
Our Ordinary Shares are currently quoted on the Nasdaq Capital Market under the symbol “GELS”. On June 27, 2025, the closing price of our Ordinary Shares was $1.81 per share.
81
EXPENSES RELATING TO THIS OFFERING
Set forth below is an itemization of the total expenses that we expect to incur in connection with this offering. With the exception of the SEC registration fee, all amounts are estimates.
|
Securities and Exchange Commission Registration Fee |
USD$ |
1,041 |
||
|
Legal Fees and Expenses |
USD$ |
150,000 |
||
|
Accounting Fees and Expenses |
USD$ |
27,000 |
||
|
Miscellaneous Expenses |
USD$ |
3,959 |
||
|
Total Expenses |
USD$ |
182,000 |
82
LEGAL MATTERS
The validity of the issuance of the shares offered in this prospectus and certain other matters of Australian law will be passed upon for us by Vistra Australia Legal Services Pty Ltd t/a Vistra Legal Australia. Ellenoff Grossman & Schole LLP, New York, New York, is acting as counsel in connection with the registration of our securities under the Securities Act, and as such, will pass upon the validity of the securities offered in this prospectus.
EXPERTS
The consolidated financial statements of Gelteq Limited for the six month period ended December 31, 2024 and for the years ended June 30, 2024 and June 30 2023 are included in this prospectus. The year ended June 30, 2024, has been audited by M&K CPAS PLLC, an independent registered public accounting firm, as set forth in their report thereon. The year ended June 30, 2023 has been audited by UHY Haines Norton, Sydney, an independent registered public accounting firm, as set forth in their report thereon. Both firms reports contain an explanatory paragraph related to substantial doubt about the ability of Gelteq Limited to continue as a going concern as described in Note 4 to the financial statements appearing elsewhere in this prospectus and are included in reliance upon such report given on the authority of such firms as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1, including relevant exhibits and schedules under the Securities Act, covering the Ordinary Shares offered by this prospectus. You should refer to our registration statements and their exhibits and schedules if you would like to find out more about us and about the Ordinary Shares. This prospectus summarizes material provisions of contracts and other documents that we refer you to. Since the prospectus may not contain all the information that you may find important, you should review the full text of these documents.
As of the date of this prospectus, we are subject to periodic reporting and other informational requirements of the Exchange Act, as applicable to foreign private issuers. Accordingly, we are required to file reports, including annual reports on Form 20-F, and other information with the SEC. As a foreign private issuer, we are exempt from the rules of the Exchange Act prescribing the furnishing and content of proxy statements to shareholders under the federal proxy rules contained in Sections 14(a), (b) and (c) of the Exchange Act, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act.
The SEC maintains a website that contains reports, proxy statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov. The information on that website is not a part of this prospectus.
83
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to incorporate by reference information into this document. This means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is considered to be a part of this document, except for any information superseded by information that is included directly in this prospectus or incorporated by reference subsequent to the date of this prospectus.
We incorporate by reference the following documents or information that we have filed with the SEC:
• our Annual Report on Form 20-F for the fiscal year ended June 30, 2024, filed with the SEC on November 15, 2024.
• our Reports of Foreign Private Issuer on Form 6-K furnished with the SEC on October 30, 2024 and March 14, 2025.
Documents incorporated by reference in this prospectus are available from us without charge upon written or oral request, excluding any exhibits to those documents that are not specifically incorporated by reference into those documents. You can obtain documents incorporated by reference in this document by requesting them from us in writing or at Gelteq Limited, Level 4, 100 Albert Road, South Melbourne VIC, 3025, Australia or via telephone at +61 3 9087 3990.
84
|
Gelteq Limited |
|
GELTEQ LIMITED
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Pages |
||
|
Interim Report of Gelteq Limited for the Six Months Period Ended December 31, 2024 and 2023 |
||
|
Consolidated statement of profit or loss and other comprehensive income |
F-2 |
|
|
F-3 |
||
|
F-4 |
||
|
F-5 |
||
|
F-6 |
||
|
F-38 |
||
|
Annual Report of Gelteq Limited for the Years Ended June 30, 2024 and 2023 |
||
|
Report of Independent Registered Public Accounting Firm — Year ended 30 June 2024 |
F-39 |
|
|
Report of Independent Registered Public Accounting Firm — Year ended 30 June 2023 |
F-41 |
|
|
F-43 |
||
|
Consolidated Statement of Financial Position as at June 30, 2024 and 2023 |
F-44 |
|
|
Consolidated Statement of Changes in Equity for Years Ended June 30, 2024 and 2023 |
F-45 |
|
|
Consolidated Statement of Cash Flows for Years Ended June 30, 2024 and 2023 |
F-46 |
|
|
F-47 |
||
|
F-95 |
F-1
|
Gelteq Limited |
|
|
Consolidated |
||||||||
|
Note |
Six months |
Six months |
||||||
|
$ |
$ |
|||||||
|
Revenue |
|
|
||||||
|
Other income |
6 |
|
|
|
|
|||
|
Revenue from contract with customer |
|
|
||||||
|
|
|
|||||||
|
Expenses |
|
|
||||||
|
Corporate expenses |
7 |
( |
) |
( |
) |
|||
|
IPO related expenses |
8 |
( |
) |
( |
) |
|||
|
Depreciation and amortisation expenses |
9 |
( |
) |
( |
) |
|||
|
Research expenses |
10 |
( |
) |
( |
) |
|||
|
Employment Expenses |
11 |
( |
) |
( |
) |
|||
|
Advertising & marketing expense |
( |
) |
( |
) |
||||
|
Consulting Fees |
( |
) |
( |
) |
||||
|
Other expenses |
( |
) |
( |
) |
||||
|
Operating loss |
( |
) |
( |
) |
||||
|
Finance costs |
12 |
( |
) |
( |
) |
|||
|
|
|
|||||||
|
Loss before income tax expense |
( |
) |
( |
) |
||||
|
Income tax expense |
13 |
|
|
|||||
|
|
|
|||||||
|
Loss after income tax expense for the period attributable to the owners of Gelteq Limited |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Other comprehensive income for the period, net of tax |
|
|
||||||
|
Total comprehensive loss for the period attributable to the owners of Gelteq Limited |
( |
) |
( |
) |
||||
|
$ |
$ |
|||||||
|
Basic loss per share |
29 |
( |
) |
( |
) |
|||
|
Diluted loss per share |
29 |
( |
) |
( |
) |
|||
The above consolidated statement of profit or loss and other comprehensive income should be read in conjunction with the accompanying notes
F-2
|
Gelteq Limited |
|
|
Consolidated |
||||||||
|
Note |
As at |
As at |
||||||
|
$ |
$ |
|||||||
|
Assets |
|
|
||||||
|
|
|
|||||||
|
Current assets |
|
|
||||||
|
Cash and cash equivalents |
14 |
|
|
|
|
|||
|
Other receivables |
|
|
|
|
||||
|
Prepayments and other assets |
16 |
|
|
|
|
|||
|
Total current assets |
|
|
|
|
||||
|
|
|
|||||||
|
Non-current assets |
|
|
||||||
|
Plant and equipment |
|
|
|
|
||||
|
Intangible assets |
17 |
|
|
|
|
|||
|
Total non-current assets |
|
|
|
|
||||
|
Total assets |
|
|
|
|
||||
|
|
|
|||||||
|
Liabilities |
|
|
||||||
|
|
|
|||||||
|
Current liabilities |
|
|
||||||
|
Trade and other payables |
18 |
|
|
|
|
|||
|
Deferred Revenue |
19 |
|
|
|
|
|||
|
Borrowings, net |
20 |
|
|
|
|
|||
|
Derivative liability |
20 |
|
|
|
||||
|
Employee benefits provisions |
|
|
|
|
||||
|
Total current liabilities |
|
|
|
|
||||
|
|
|
|||||||
|
Non-current liabilities |
|
|
||||||
|
Borrowings |
20 |
|
|
|
|
|||
|
Employee benefits provisions |
|
|
|
|
||||
|
Total non-current liabilities |
|
|
|
|
||||
|
Total liabilities |
|
|
|
|
||||
|
Net assets |
|
|
|
|
||||
|
|
|
|||||||
|
Equity |
|
|
||||||
|
Issued capital |
21 |
|
|
|
|
|||
|
Accumulated losses |
( |
) |
( |
) |
||||
|
Total equity |
|
|
|
|
||||
The above consolidated statement of financial position should be read in conjunction with the accompanying notes
F-3
|
Gelteq Limited |
|
|
Consolidated |
Issued |
Reserve |
Accumulated |
Total |
||||||
|
$ |
$ |
$ |
$ |
|||||||
|
Balance at 1 July 2023 |
|
( |
) |
|
|
|||||
|
|
|
|||||||||
|
Loss after income tax expense for the period |
( |
) |
( |
) |
||||||
|
Other comprehensive income for the period, net of tax |
|
|
||||||||
|
Total comprehensive loss for the period |
( |
) |
( |
) |
||||||
|
Balance at 31 December 2023 |
|
( |
) |
|
|
|||||
|
Consolidated |
Issued |
Reserve |
Accumulated |
Total |
||||||
|
$ |
$ |
$ |
$ |
|||||||
|
Balance at 1 July 2024 |
|
( |
) |
|
|
|||||
|
Loss after income tax expense for the period |
( |
) |
( |
) |
||||||
|
Other comprehensive income for the period, net of tax |
|
|
||||||||
|
Total comprehensive loss for the period |
( |
) |
( |
) |
||||||
|
|
|
|||||||||
|
Transactions with owners in their capacity as owners: |
|
|
||||||||
|
Contributions of equity, net of transaction costs (note 21) |
|
|
|
|
||||||
|
Balance at 31 December 2024 |
|
( |
) |
|
|
|||||
The above consolidated statement of changes in equity should be read in conjunction with the accompanying notes
F-4
|
Gelteq Limited |
|
|
Consolidated |
||||||||
|
Note |
December |
December |
||||||
|
$ |
$ |
|||||||
|
Cash flows from operating activities |
|
|
||||||
|
Payments to suppliers and employees (inclusive of GST) |
( |
) |
( |
) |
||||
|
Research & development tax incentives |
|
|
|
|||||
|
Receipt from Customers |
|
|
|
|||||
| ( |
) |
( |
) |
|||||
|
Interest and other finance costs paid |
( |
) |
( |
) |
||||
|
Net cash used in operating activities |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Cash flows from investing activities |
|
|
||||||
|
Payment towards procurement of property, plant and equipment |
( |
) |
|
|||||
|
Payment towards acquisition of intangibles |
( |
) |
( |
) |
||||
|
Net cash used in investing activities |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Cash flows from financing activities |
|
|
||||||
|
Proceeds from issue of shares |
21 |
|
|
|
||||
|
Proceeds from borrowings |
|
|
|
|
||||
|
Capital issue costs |
( |
) |
|
|||||
|
Repayment of lease liabilities |
|
( |
) |
|||||
|
Net cash from financing activities |
|
|
|
|
||||
|
|
|
|||||||
|
Net increase/(decrease) in cash and cash equivalents |
|
|
( |
) |
||||
|
Cash and cash equivalents at the beginning of the financial half-year |
|
|
|
|
||||
|
Effects of exchange rate changes on cash and cash equivalents |
|
|
|
|||||
|
Cash and cash equivalents at the end of the financial half-year |
|
|
|
|
||||
The above consolidated statement of cash flows should be read in conjunction with the accompanying notes
F-5
|
Gelteq Limited |
|
Note 1. General information
The condensed consolidated financial statements covers Gelteq Limited (“Gelteq” or the “Company”) and its controlled entities (referred to herein as the “Consolidated Entity”). Gelteq Limited is a Company limited by shares, incorporated and domiciled in Australia.
The condensed consolidated financial statements are presented in Australian dollars, which is Gelteq Limited’s functional and presentation currency.
The principal activities of the consolidated entity during the periods ended 31 December 2024 and 31 December 2023 (financial period(s)) were the development and testing of a gel-based delivery system for humans.
The names of the directors in office at any time during or since the end of the financial period are:
|
Simon Szewach (Executive Chairman)* |
|
Nathan Jacob. Givoni (Executive Director) |
|
Jeff Olyniec (Non-Executive Director) |
|
Philip Dalidakis (Non-Executive Director) |
|
Prof David Morton (Non-Executive Director) (Resigned on 30 April 2025) |
____________
* Mr. Simon resigned as an executive Chairman on 31 March 2025 and remains as a non-executive Chairman as at the date of this report.
The directors have been in office since the start of the financial period to the date of this report unless otherwise stated.
The condensed consolidated financial statements were authorised for issue, in accordance with a resolution of directors, on 30 June, 2025.
Note 2. Basis of preparation
The condensed consolidated financial statements are presented in Australian Dollars, which is also the Consolidated Entity’s functional currency. Amounts are rounded to the nearest dollar, unless otherwise stated.
These condensed consolidated financial statements have been prepared in accordance with International Financial Reporting Standards and International Accounting Standards as issued by the International Accounting Standards Board (IASB) and Interpretations (collectively IFRSs).
The preparation of condensed consolidated financial statements in compliance with adopted IFRS requires the use of certain critical accounting estimates. It also requires the Consolidated Entity’s management to exercise judgment in applying the Consolidated Entity’s accounting policies. The areas where significant judgments and estimates have been made in preparing the condensed consolidated financial statements and their effect are disclosed in note 4.
Basis of measurement
The condensed consolidated financial statements have been prepared on a historical cost basis.
These general purpose condensed consolidated financial statements for the reporting periods ended 31 December 2024 and 30 June 2024 have been prepared in accordance with International Accounting Standards IAS 34 ‘Interim Financial Reporting’ as appropriate for for-profit oriented entities.
These general purpose condensed consolidated financial statements do not include all the notes of the type normally included in annual financial statements. Accordingly, where appropriate these condensed consolidated financial statements are to be read in conjunction with the annual report for the year ended 31 December 2024.
The principal accounting policies adopted are consistent with those of the previous financial year and corresponding interim reporting period, unless otherwise stated.
F-6
|
Gelteq Limited Note 2. Basis of preparation (cont.) |
|
New standards, interpretations and amendments effective — December 2024
The Consolidated Entity has adopted all of the new or amended Accounting Standards and Interpretations issued by the International Accounting Standards Board (IASB) that are mandatory for the current reporting period.
New standards, interpretations and amendments not yet effective — December 2024
There are a number of standards, amendments to standards, and interpretations which have been issued by the IASB that are effective in future accounting periods that the Consolidated Entity has decided not to adopt early.
The following amendments to standards are applicable to the Company and effective for future reporting periods:
|
IFRS18 Presentation and Disclosure in Financial Statements This standard is applicable to annual reporting periods beginning on or after 1 January 2027. The standard replaces IAS 1 Presentation of Financial Statements, with many of the original disclosure requirements retained and there will be no impact on the recognition and measurement of items in the financial statements. But the standard will affect presentation and disclosure in the financial statements, including introducing five categories in the statement of profit or loss and other comprehensive income: operating, investing, financing, income taxes and discontinued operations. The standard introduces two mandatory sub-totals in the statement: ‘Operating profit’ and ‘Profit before financing and income taxes’. There are also new disclosure requirements for ‘management-defined performance measures’, such as earnings before interest, taxes, depreciation and amortisation (‘EBITDA’) or ‘adjusted profit’. The standard provides enhanced guidance on grouping of information (aggregation and disaggregation), including whether to present this information in the primary financial statements or in the notes. The Consolidated Entity will adopt this standard from 1 July 2027. As at reporting date, the Consolidated Entity has not completed an assessment on the impact of the standard, but it is expected that there will be a material change to the layout of the statement of profit or loss and other comprehensive income. Amendments to IAS 21 — Lack of Exchangeability The amendments are applicable to annual reporting periods beginning on or after 1 January 2025. The Standard amends IAS 21 and IFRS 1 to require entities to apply a consistent approach to determining whether a currency is exchangeable into another currency and the spot exchange rate to use when it is not exchangeable. New disclosures are required to help users assess the impact of using an estimated exchange rate on the financial statements. The Consolidated Entity will adopt this standard from its application date and where appropriate incorporate the additional disclosures required. |
These standards are not expected to have a material impact on the Consolidated Entity in the current or future reporting periods and on foreseeable future transactions. However, management will continue to assess this closer to the application date of each standard.
Other
The Consolidated Entity does not expect any other standards issued by the IASB, but not yet effective, to have a material impact on the Consolidated Entity.
(a) Principles of consolidation
The condensed consolidated financial statements incorporate the assets and liabilities of all subsidiaries of Gelteq Limited (‘Company’ or ‘Parent entity’) as at 31 December 2024 and the results of all subsidiaries for the period ended 31 December 2024 and the period ended 31 December 2023 . Gelteq Limited and its subsidiaries together are referred to in these condensed consolidated financial statements as the ‘Consolidated Entity’.
F-7
|
Gelteq Limited Note 2. Basis of preparation (cont.) |
|
Subsidiaries are all those entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. They are de-consolidated from the date that control ceases.
Intercompany transactions, balances and unrealised gains on transactions between entities in the Company are eliminated. Unrealised losses are also eliminated unless the transaction provides evidence of the impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Company.
The acquisition of subsidiaries is accounted for using the asset acquisition method of accounting. A change in ownership interest, without the loss of control, is accounted for as an equity transaction, where the difference between the consideration transferred and the book value of the share of the non-controlling interest acquired is recognised directly in equity attributable to the parent.
Where the Company loses control over a subsidiary, it derecognises the assets including goodwill, liabilities and non-controlling interest in the subsidiary together with any cumulative translation differences recognised in equity. The Company recognises the fair value of the consideration received and the fair value of any investment retained together with any gain or loss in profit or loss.
Note 3. Summary of significant accounting policies
The principal accounting policies adopted in the preparation of the consolidated financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
(a) Revenue from contracts with customers
Revenue arises mainly from the manufacturing and sale of products. To determine whether to recognise revenue, the Consolidated Entity follows a 5-step process:
(1) Identifying the contract with a customer
(2) Identifying the performance obligations
(3) Determining the transaction price
(4) Allocating the transaction price to the performance obligations
(5) Recognising revenue when/as the performance obligations are satisfied.
Revenue is recognised either at a point in time or over time, when the Consolidated Entity satisfies performance obligations by transferring the promised goods or services to its customers.
The Consolidated Entity recognises contract liabilities for consideration received in respect to unsatisfied performance obligations and reports these amounts as other liabilities (which we refer to as deferred revenues) in the condensed consolidated statement of financial position. Similarly, if the Consolidated Entity satisfies a performance obligation before it receives the consideration, the Consolidated Entity recognises either a contract asset or a receivable in its condensed consolidated statement of financial position, depending on whether something other than the passage of time is required before the consideration is due.
Sale of Products
Revenue from sale of product for a fixed fee is recognised when or as the Consolidated Entity transfers control of the assets to the customer.
F-8
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
(b) Research and Development Tax Incentive
The Research and Development Tax Incentive programme provides tax offsets for expenditure on eligible R&D activities. Under the programme, the Consolidated Entity, is entitled to a refundable R&D credit in Australia on the eligible R&D expenditure incurred on eligible R&D activities. The refundable R&D tax offset is accounted for under IAS 20 Accounting for Government Grants and Disclosure of Government Assistance, as per which the R&D tax offset income is recognised when there is reasonable assurance that it will be received. It is recognised in the condensed consolidated statement of comprehensive income in the same period that the related costs are recognised as expenses and relates to refundable amounts on approved expenses.
(c) Business Combinations/Asset Acquisitions
Business combinations occur where an acquirer obtains control over one or more businesses and results in the consolidation of its assets and liabilities.
A business combination is accounted for by applying the acquisition method, unless it is a combination involving entities or businesses under common control. The business combination will be accounted for from the date that control is obtained, whereby the fair value of the identifiable assets acquired and liabilities (including contingent liabilities) assumed are recognised (subject to certain limited exceptions).
If the acquisition of an asset or a group of assets does not constitute a business, the individual identifiable assets acquired (including intangible assets) and liabilities are assumed. The cost of the group shall be allocated to the individual identifiable assets and liabilities on the basis of their relative fair values at the date of purchase. Such a transaction or event does not give rise to goodwill.
Determining whether a particular set of assets and activities is a business should be based on whether the integrated set is capable of being conducted and managed as a business by a market participant. Thus, in evaluating whether a particular set is a business, it is not relevant whether a seller operated the set as a business or whether the acquirer intends to operate the set as a business. In the absence of evidence to the contrary, a particular set of assets and activities in which goodwill is present shall be presumed to be a business. However, a business need not have goodwill.
(d) Income Tax
The income tax expense (income) for the periods ended 31 December 2024 and 31 December 2023 comprises current income tax expense (income) and deferred tax expense (income).
Current tax assets and liabilities are offset where a legally enforceable right of set-off exists and it is intended that net settlement or simultaneous realisation and settlement of the respective asset and liability will occur. Deferred tax assets and liabilities are offset where: (a) a legally enforceable right of set-off exists; and (b) the deferred tax assets and liabilities relate to income taxes levied by the same taxation authority on either the same taxable entity or different taxable entities where it is intended that net settlement or simultaneous realisation and settlement of the respective asset and liability will occur in future periods in which significant amounts of deferred tax assets or liabilities are expected to be recovered or settled.
Deferred income tax expense reflects movements in deferred tax asset and deferred tax liability balances during the period, as well as unused tax losses.
Current and deferred income tax expense (income) is charged or credited outside profit or loss when the tax relates to items that are recognised outside profit or loss or arising from a business combination.
Except for business combinations, no deferred income tax is recognised from the initial recognition of an asset or liability where there is no effect on accounting or taxable profit or loss.
F-9
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
A deferred tax liability shall be recognised for all taxable temporary differences, except to the extent that the deferred tax liability arises from:
(a) the initial recognition of goodwill; or
(b) the initial recognition of an asset or liability in a transaction which:
(i) is not a business combination; and
(ii) at the time of the transaction, affects neither accounting profit nor taxable profit (tax loss).
Deferred tax assets and liabilities are calculated at the tax rates that are expected to apply to the period when the asset is realised or the liability is settled and their measurement also reflects the manner in which management expects to recover or settle the carrying amount of the related asset or liability.
Deferred tax assets relating to temporary differences and unused tax losses are recognised only to the extent that it is probable that future taxable profit will be available against which the benefits of the deferred tax asset can be utilised.
(e) Fair Value of Assets and Liabilities
The Consolidated Entity measures some of its assets and liabilities at fair value on either a recurring or non-recurring basis, depending on the requirements of the applicable Accounting Standard.
Fair value is the price the Consolidated Entity would receive to sell an asset or would have to pay to transfer a liability in an orderly (i.e. unforced) transaction between independent, knowledgeable and willing market participants at the measurement date.
As fair value is a market-based measure, the closest equivalent observable market pricing information is used to determine fair value. Adjustments to market values may be made having regard to the characteristics of the specific asset or liability. The fair values of assets and liabilities that are not traded in an active market are determined using one or more valuation techniques. These valuation techniques maximise, to the extent possible, the use of observable market data.
To the extent possible, market information is extracted from either the principal market for the asset or liability (i.e. the market with the greatest volume and level of activity for the asset or liability) or, in the absence of such a market, the most advantageous market available to the entity at the end of the reporting period (i.e. the market that maximises the receipts from the sale of the asset or minimises the payments made to transfer the liability, after taking into account transaction costs and transport costs).
For non-financial assets, the fair value measurement also takes into account a market participant’s ability to use the asset in its highest and best use or to sell it to another market participant that would use the asset in its highest and best use.
The fair value of liabilities and the entity’s own equity instruments (excluding those related to share-based payment arrangements) may be valued, where there is no observable market price in relation to the transfer of such financial instruments, by reference to observable market information where such instruments are held as assets. Where this information is not available, other valuation techniques are adopted and, where significant, are detailed in the respective note to the condensed consolidated financial statements.
(f) Financial Instruments
Initial recognition and measurement
Financial assets and financial liabilities are recognised when the entity becomes a party to the contractual provisions of the instrument. For financial assets, this is equivalent to the date that the Consolidated Entity commits itself to either purchase or sell the asset (i.e. trade date accounting is adopted).
F-10
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Financial instruments (except for trade receivables) are initially measured at fair value plus transactions costs, except where the instrument is classified ‘at fair value through profit or loss’ in which case transactions costs are recognised as expenses in profit or loss immediately. Where available, quoted prices in an active market are used to determine fair value. In other circumstances, valuation techniques are adopted.
Trade receivables are initially measured at the transaction price if the trade receivables do not contain a significant financing component or if the practical expedient was applied as specified in IFRS 15: Revenue from Contracts with Customers.
Classification and subsequent measurement
Financial liabilities
Financial liabilities are subsequently measured at:
– amortised cost; or
– fair value through profit and loss.
A financial liability is measured at fair value through profit and loss if the financial liability is:
– a contingent consideration of an acquirer in a business combination to which IFRS 3: Business Combinations applies;
– held for trading; or
– initially designated as at fair value through profit or loss.
All other financial liabilities are subsequently measured at amortised cost using the effective interest method. The effective interest method is a method of calculating the amortised cost of a debt instrument and of allocating interest expense to profit or loss over the relevant period.
The effective interest rate is the internal rate of return of the financial asset or liability. That is, it is the rate that exactly discounts the estimated future cash flows through the expected life of the instrument to the net carrying amount at initial recognition.
Any gains or losses arising on changes in fair value are recognised in profit or loss to the extent that they are not part of a designated hedging relationship.
The change in fair value of the financial liability attributable to changes in the issuer’s credit risk is taken to other comprehensive income and is not subsequently reclassified to profit or loss. Instead, it is transferred to retained earnings upon derecognition of the financial liability.
If taking the change in credit risk to other comprehensive income enlarges or creates an accounting mismatch, these gains or losses should be taken to profit or loss rather than other comprehensive income. A financial liability cannot be reclassified.
Financial assets
Financial assets are subsequently measured at:
– amortised cost;
– fair value through other comprehensive income; or
– fair value through profit or loss.
F-11
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Measurement is on the basis of two primary criteria:
– the contractual cash flow characteristics of the financial asset; and
– the business model for managing the financial assets.
A financial asset that meets the following conditions is subsequently measured at amortised cost:
– the financial asset is managed solely to collect contractual cash flows; and
– contractual terms within the financial asset give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding on specified dates.
A financial asset that meets the following conditions is subsequently measured at amortised cost:
– the financial asset is managed solely to collect contractual cash flows; and
– the contractual terms within the financial asset give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding on specified dates.
A financial asset that meets the following conditions is subsequently measured at fair value through other comprehensive income:
– the contractual terms within the financial asset give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding on specified dates; and
– the business model for managing the financial asset comprises both contractual cash flows collection and the selling of the financial asset.
By default, all other financial assets that do not meet the measurement conditions of amortised cost and fair value through other comprehensive income are subsequently measured at fair value through profit or loss.
The Consolidated Entity initially designates a financial instrument as measured at fair value through profit or loss if:
• it eliminates or significantly reduces a measurement or recognition inconsistency (often referred to as an “accounting mismatch”) that would otherwise arise from measuring assets or liabilities or recognising the gains and losses on them on different bases;
• it is in accordance with the documented risk management or investment strategy and information about the groupings is documented appropriately, so the performance of the financial liability that is part of a group of financial liabilities or financial assets can be managed and evaluated consistently on a fair value basis; and
• it is a hybrid contract that contains an embedded derivative that significantly modifies the cash flows otherwise required by the contract.
The initial measurement of financial instruments at fair value through profit or loss is a one-time option on initial classification and is irrevocable until the financial asset is derecognised.
Derecognition
Derecognition of financial liabilities
A liability is derecognised when it is extinguished (i.e. when the obligation in the contract is discharged, cancelled or expires). An exchange of an existing financial liability for a new one with substantially modified terms, or a substantial modification to the terms of a financial liability, is treated as an extinguishment of the existing liability and recognition of a new financial liability.
F-12
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
The difference between the carrying amount of the financial liability derecognised and the consideration paid and payable, including any non-cash assets transferred or liabilities assumed, is recognised in profit or loss.
Derecognition of financial assets
A financial asset is derecognised when the holder’s contractual rights to its cash flows expires, or the asset is transferred in such a way that all the risks and rewards of ownership are substantially transferred.
All the following criteria need to be satisfied for the derecognition of a financial asset:
• the right to receive cash flows from the asset has expired or been transferred;
• all risk and rewards of ownership of the asset have been substantially transferred; and
• the Consolidated Entity no longer controls the asset (i.e it has no practical ability to make unilateral decisions to sell the asset to a third party).
On derecognition of a financial asset measured at amortised cost, the difference between the asset’s carrying amount and the sum of the consideration received and receivable is recognised in profit or loss.
On derecognition of a debt instrument classified as fair value through other comprehensive income, the cumulative gain or loss previously accumulated in the investment revaluation reserve is reclassified to profit or loss.
Convertible notes payable
Convertible notes payable are financial instruments which contain a separate financial liability and equity instrument. These financial instruments are accounted for separately dependent on the nature of their components. The identification of such components embedded within a convertible notes payable requires significant judgement given that it is based on the interpretation of the substance of the contractual arrangement. The convertible notes are considered to contain embedded derivatives. The embedded derivatives were measured at fair value upon initial recognition based on a Black-Scholes valuation model and separated from the debt component of the notes. The debt component of the notes is measured at residual value upon initial recognition. Subsequent to initial recognition, the embedded derivative components are re-measured at fair value at each reporting date while the debt components are accreted to the face value of the note using the effective interest rate through periodic charges to finance expense over the term of the note.
In accordance with IFRS 9, where an indeterminate number of shares may be issued in due course upon the conversion of the convertible notes, or the convertible notes are convertible at a discount to market, the embedded derivative is accounted for as a liability.
(g) Impairment of assets
At the end of each reporting period, the Consolidated Entity assesses whether there is any indication that an asset may be impaired. The assessment will include considering external sources of information and internal sources of information, including dividends received from subsidiaries, associates or joint ventures deemed to be out of pre-acquisition profits. If such an indication exists, an impairment test is carried out on the asset by comparing the recover able amount of the asset, being the higher of the asset’s fair value less costs to sell and value in use to the asset’s carrying amount. Any excess of the asset’s carrying amount over its recoverable amount is recognised immediately in profit or loss, unless the asset is carried at a revalued amount in accordance with another Standard. Any impairment loss of a revalued asset is treated as a revaluation decrease in accordance with that other Standard.
Where it is not possible to estimate the recoverable amount of an individual asset, the Consolidated Entity estimates the recoverable amount of the cash-generating unit to which the asset belongs.
F-13
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Impairment testing is performed annually for goodwill and intangible assets with indefinite lives.
When an impairment loss subsequently reverses, the carrying amount of the asset (or cash-generating unit) is increased to the revised estimate of its recoverable amount, but so that the increased carrying amount does not exceed the carrying amount that would have been determined had no impairment loss been recognised for the asset (or cash-generating unit) in prior years. A reversal of an impairment loss is recognised immediately in profit or loss, unless the relevant asset is carried at a revalued amount, in which case the reversal of the impairment loss is treated as a revaluation increase.
(h) Inventories
Raw materials, work in progress and finished goods are stated at the lower of cost and net realisable value on a ‘first in first out’ basis. Cost comprises of direct materials and delivery costs, direct labour, import duties and taxes, an appropriate proportion of variable and fixed overhead expenditure based on normal operating capacity. Costs of purchased inventory are determined after deducting rebates and discounts received or receivable.
Raw materials, finished goods and work in progress are stated at the lower of cost and net realisable value. Cost comprises of purchase and delivery costs, net of rebates and discounts received or receivable. Costs are assigned to individual items of inventory on the ‘first in first out’ basis.
Net realisable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
(i) Right-of-use assets
A right-of-use asset is recognised at the commencement date of a lease. The right-of-use asset is measured at cost, which comprises the initial amount of the lease liability, adjusted for, as applicable, any lease payments made at or before the commencement date net of any lease incentives received, any initial direct costs incurred, and, except where included in the cost of inventories, an estimate of costs expected to be incurred for dismantling and removing the underlying asset, and restoring the site or asset.
Right-of-use assets are depreciated on a straight-line basis over the unexpired period of the lease or the estimated useful life of the asset, whichever is the shorter. Where the Consolidated Entity expects to obtain ownership of the leased asset at the end of the lease term, the depreciation is over its estimated useful life. Right-of use assets are subject to impairment or adjusted for any remeasurement of lease liabilities.
The Consolidated Entity has elected not to recognise a right-of-use asset and corresponding lease liability for short-term leases with terms of 12 months or less and leases of low-value assets. Lease payments on these assets are expensed to profit or loss as incurred.
(j) Intangible Assets Other than Goodwill
Trade secrets
Trade secrets with finite useful lives that are acquired separately, including those acquired in a business combination recognised separately from goodwill, are carried at cost less accumulated amortisation and accumulated impairment losses. Amortisation is recognised on a straight-line basis over their estimated useful lives which are disclosed below. The estimated useful life and amortisation method are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
Research and development
Expenditure during the research phase of a project is recognised as an expense when incurred.
F-14
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Under IFRS 138, An intangible asset arising from development (or from the development phase of an internal project) shall be recognised if, and only if, an entity can demonstrate all of the following:
(a) the technical feasibility of completing the intangible asset so that it will be available for use or sale.
(b) its intention to complete the intangible asset and use or sell it.
(c) its ability to use or sell the intangible asset.
(d) how the intangible asset will generate probable future economic benefits. Among other things, the entity can demonstrate the existence of a market for the output of the intangible asset or the intangible asset itself or, if it is to be used internally, the usefulness of the intangible asset.
(e) the availability of adequate technical, financial and other resources to complete the development and to use or sell the intangible asset.
(f) its ability to measure reliably the expenditure attributable to the intangible asset during its development.
Development expenditure that does not meet the criteria for capitalisation above are recognised as an expense as incurred.
Patents & trademarks
Patents and trademarks are measured initially at purchase cost and are amortised on a straight line basis over their estimated useful lives.
The amortisation rates used for each class of intangible asset with a finite useful life are:
| Class of Intangible Asset | Amortisation | |
| Trade Secrets | | |
| Patents and Trademarks | |
Foreign Currency Transactions and Balances
(k) Functional and presentation currency
The functional currency of each of the companies in the Consolidated Entity is measured using the currency of the primary economic environment in which that Company operates. The condensed consolidated financial statements are presented in Australian dollars, which is the Parent company’s functional currency.
Transactions and balances
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transaction. Foreign currency monetary items are translated at the period-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined.
Exchange differences arising on the translation of monetary items are recognised in profit or loss, except where deferred in equity as a qualifying cash flow or net investment hedge.
Exchange differences arising on the translation of non-monetary items are recognised directly in other comprehensive income to the extent that the underlying gain or loss is directly recognised in other comprehensive income; otherwise the exchange difference is recognised in profit or loss.
F-15
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
(l) Employee Benefit Provisions
Short-term obligations
Liabilities for accumulating annual leave that are expected to be settled wholly within 12 months after the end of the period in which the employees render the related service are recognised in respect of employees’ services up to the end of the reporting period and are measured at the amounts expected to be paid when the liabilities are settled. The liabilities are presented as current employee benefit obligations in the balance sheet.
Other long-term employee benefit obligations
The liabilities for long service leave are not expected to be settled wholly within 12 months after the end of the period in which the employees render the related service. They are therefore measured as the present value of expected future payments to be made in respect of services provided by employees up to the end of the reporting period using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service.
The obligations are presented as current liabilities in the balance sheet if the entity does not have an unconditional right to defer settlement for at least twelve months after the reporting date, regardless of when the actual settlement is expected to occur.
(m) Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, deposits held at call with banks, other short-term highly liquid investments with original maturities of three months or less, and bank overdrafts.
(n) Government Grants
Government grants received on capital expenditure are generally deducted in arriving at the carrying amount of the asset purchased. Grants for revenue expenditure are recognised as other income by the Consolidated Entity. Where retention of a government grant is dependent on the Consolidated Entity satisfying certain criteria, it is initially recognised as deferred income. When the criteria for retention have been satisfied, the deferred income balance is released to the condensed consolidated statement of comprehensive income or netted against the asset purchased.
(o) Trade and other receivables
Trade and other receivables are recognised at amortised cost, less any allowance for expected credit losses.
(p) Trade and Other Payables
Trade and other payables represent the liabilities for goods and services received by the entity that remain unpaid at the end of the reporting period. The balance is recognised as a current liability with the amounts normally paid within 30 days of recognition of the liability.
Trade and other payables are initially measured their fair value and subsequently measured at amortised cost using the effective interest method.
Accruals are recognised when they can be reasonably estimated and attributed to the relevant financial period. They are assessed for fair value and carried at amortised cost. They are derecognised when a liability for payment is raised as a trade or other payable.
F-16
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
(q) Borrowings
Borrowings are initially recognised at fair value, net of transaction costs incurred. Borrowings are subsequently measured at amortised cost. Any difference between the proceeds (net of transaction costs) and the redemption amount is recognised in profit or loss over the year of the borrowings using the effective interest method.
Borrowings are removed from the balance sheet when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial liability that has been extinguished or transferred to another party and the consideration paid, including any non-cash assets transferred or liabilities assumed, is recognised in profit or loss as other income or finance costs.
Borrowings are classified as current liabilities unless the Consolidated Entity has an unconditional right to defer settlement of the liability for at least 12 months after the reporting period.
Borrowing Costs
Borrowing costs directly attributable to the acquisition, construction or production of assets that necessarily take a substantial period of time to prepare for their intended use or sale are added to the cost of those assets, until such time as the assets are substantially ready for their intended use or sale.
All other borrowing costs are recognised in profit or loss in the period in which they are incurred.
(r) Lease liabilities
A lease liability is recognised at the commencement date of a lease. The lease liability is initially recognised at the present value of the lease payments to be made over the term of the lease, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, the Consolidated Entity’s incremental borrowing rate. Lease payments comprise of fixed payments less any lease incentives receivable, variable lease payments that depend on an index or a rate, amounts expected to be paid under residual value guarantees, exercise price of a purchase option when the exercise of the option is reasonably certain to occur, and any anticipated termination penalties. The variable lease payments that do not depend on an index or a rate are expensed in the period in which they are incurred.
Lease liabilities are measured at amortised cost using the effective interest method. The carrying amounts are remeasured if there is a change in the following: future lease payments arising from a change in an index or a rate used; residual guarantee; lease term; certainty of a purchase option and termination penalties. When a lease liability is remeasured, an adjustment is made to the corresponding right-of use asset, or to profit or loss if the carrying amount of the right-of-use asset is fully written down.
(s) Goods and Services Tax (GST)
Revenues, expenses and assets are recognised net of the amount of GST, except where the amount of GST incurred is not recoverable from the Australian Taxation Office (ATO).
Receivables and payables are stated inclusive of the amount of GST receivable or payable. The net amount of GST recoverable from, or payable to, the ATO is included with other receivables or payables in the statement of financial position.
(t) Earnings per Share (EPS)
Basic loss per share
Basic earnings per share is calculated by dividing the profit attributable to equity holders of the Consolidated Entity, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the period, adjusted for bonus elements in ordinary shares issued during the period.
F-17
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Diluted loss per share
Diluted loss per share adjusts the figures used in the determination of basic loss per share to take into account the after income tax effect of interest and other financing costs associated with dilutive potential ordinary shares and the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares, unless anti dilutive.
(u) Operating segments
Operating segments are presented using the ‘management approach’, where the information presented is on the same basis as the internal reports provided to the Chief Operating Decision Makers (‘CODM’). The CODM is responsible for the allocation of resources to operating segments and assessing their performance.
(v) Share-based payments
Equity-settled and cash-settled share-based compensation benefits are provided to employees.
Equity-settled transactions
Equity-settled transactions are awards of shares, or options over shares, that are provided to employees in exchange for the rendering of services. Cash-settled transactions are awards of cash for the exchange of services, where the amount of cash is determined by reference to the share price.
The cost of equity-settled transactions are measured at fair value on grant date. Fair value is generally determined using either the Binomial or Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk free interest rate for the term of the option, together with non-vesting conditions that do not determine whether the Consolidated Entity receives the services that entitle the employees to receive payment. No account is taken of any other vesting conditions. There are no such equity settled transactions where fair value is measured under these methods for financial current or previous reporting periods.
The cost of equity-settled transactions are recognised as an expense with a corresponding increase in equity over the vesting period. The cumulative charge to profit or loss is calculated based on the grant date fair value of the award, the best estimate of the number of awards that are likely to vest and the expired portion of the vesting period. The amount recognised in profit or loss for the period is the cumulative amount calculated at each reporting date less amounts already recognised in previous periods.
Cash-settled transactions
The cost of cash-settled transactions is initially, and at each reporting date until vested, determined by applying either the Binomial or Black-Scholes option pricing model, taking into consideration the terms and conditions on which the award was granted. The cumulative charge to profit or loss until settlement of the liability is calculated as follows:
• during the vesting period, the liability at each reporting date is the fair value of the award at that date multiplied by the expired portion of the vesting period.
• from the end of the vesting period until settlement of the award, the liability is the full fair value of the liability at the reporting date.
All changes in the liability are recognised in profit or loss. The ultimate cost of cash-settled transactions is the cash paid to settle the liability.
There are no cash settled transactions for period ended 31 March 2024, or the period ended 31st December 2024 or the 2024 financial period.
F-18
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Market conditions are taken into consideration in determining fair value. Therefore, any awards subject to market conditions are considered to vest irrespective of whether or not that market condition has been met, provided all other conditions are satisfied.
If equity-settled awards are modified, as a minimum an expense is recognised as if the modification has not been made. An additional expense is recognised, over the remaining vesting period, for any modification that increases the total fair value of the share-based compensation benefit as at the date of modification.
If the non-vesting condition is within the control of the Consolidated Entity or employee, the failure to satisfy the condition is treated as a cancellation. If the condition is not within the control of the Consolidated Entity or employee and is not satisfied during the vesting period, any remaining expense for the award is recognised over the remaining vesting period, unless the award is forfeited.
If equity-settled awards are cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognised immediately. If a new replacement award is substituted for the cancelled award, the cancelled and new award is treated as if they were a modification.
(w) Comparative Figures
When required by Accounting Standards, comparative figures have been adjusted to conform to changes in presentation for the current financial period.
Where the Consolidated Entity retrospectively applies an accounting policy, makes a retrospective restatement or reclassifies items in its financial statements, a third statement of financial position as at the beginning of the preceding period in addition to the minimum comparative condensed consolidated financial statements is presented
Note 4. Critical accounting judgements, estimates and assumptions
The preparation of the condensed consolidated financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the condensed consolidated financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results. The judgements, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities (refer to the respective notes) within the next financial year are discussed below.
Impacts of Covid-19
Judgement has been exercised in considering the impacts that the Coronavirus (COVID-19) pandemic has had, or may have, on the consolidated entity based on known information. This consideration extends to the nature of the products and services offered, customers, supply chain, staffing and geographic regions in which the consolidated entity operates. Other than as addressed in specific notes, there does not currently appear to be either any significant impact upon the condensed consolidated financial statements or any significant uncertainties with respect to events or conditions which may impact the consolidated entity unfavorably as at the reporting date or subsequently as a result of the Coronavirus (COVID-19) pandemic.
Estimation of useful lives of assets
The consolidated entity determines the estimated useful lives and related depreciation and amortisation charges for its property, plant and equipment and finite life intangible assets. The useful lives could change significantly as a result
F-19
|
Gelteq Limited Note 4. Critical accounting judgements, estimates and assumptions (cont.) |
|
of technical innovations or some other event. The depreciation and amortization charge will increase where the useful lives are less than previously estimated lives, or technically obsolete or non-strategic assets that have been abandoned or sold will be written off or written down.
Intangible assets
The Consolidated Entity tests annually, or more frequently if events or changes in circumstances indicate impairment, whether indefinite life or finite life intangible assets have suffered any impairment, in accordance with the accounting policy stated in note 3. The recoverable amounts of cash-generating units have been determined based on value-in-use calculations. These calculations require the use of assumptions, including estimated discount rates based on the current cost of capital and growth rates of the estimated future cash flows.
Going Concern
The working capital position as at 31 December 2024 of the Consolidated Entity results in an excess of current liabilities over current assets of $
The above matters give rise to a material uncertainty that may cast significant doubt over the Consolidated Entity’s ability to continue as a going concern. Therefore, the Consolidated Entity may be unable to realise its assets and discharge its liabilities in the normal course of business at the amounts stated in the consolidated financial statements. Notwithstanding the above matters, the Directors believe that it is reasonably foreseeable that the Consolidated Entity will be able to continue as a going concern and that it is appropriate to adopt the going concern basis in the preparation of the financial report, after considering the following matters:
• The directors have prepared detailed cash flow projections for a period of at least 12 months from the date of signing this consolidated financial report.
• The Consolidated Entity’s ability to fund its operations is dependent upon management’s plans and execution, which include raising additional capital, if required, through public and other offerings, obtaining regulatory approvals for its products and generating revenues from these products and having the ability to be able to reduce expenditure accordingly if required, in order to be able to pay its debts as and when they fall due.
• On 21 February, 2025 the Consolidated Entity’s board of directors approved by resolution a raising of up to AUD$
• On 13 March, 2025, the Consolidated Entity signed a purchase agreement with Lincoln Park Capital Fund, LLC (“Lincoln Park”) for an Equity Line of Credit, whereby we may receive gross proceeds of up to USD$
The Consolidated Entity’s six month condensed consolidated financial statements have therefore been prepared on a going concern basis which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. The six month condensed consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities should the Consolidated Entity be unable to continue as a going concern.
F-20
|
Gelteq Limited |
|
Note 5. Operating segments
During the current financial period, the Consolidated Entity operated in
IFRS 8 requires operating segments to be identified on the basis of internal reports about the components of the Consolidated Entity that are regularly reviewed by the chief operating decision maker in order to allocate resources to the segment and to assess its performance. In the current year the board reviews the Consolidated Entity as
Note 6. Other income
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Net foreign exchange gain |
|
|
||
|
Research & Development – tax incentive |
|
|
||
|
Other income |
|
|
||
Note 7. Corporate expenses
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Accounting expense |
|
|
||
|
Professional Fees |
|
|
||
|
Management Fees |
|
|||
|
Audit fees |
|
|||
|
Entertainment |
|
|||
|
Insurance |
|
|||
|
Investor Relation |
|
|||
|
Public Relation Fee |
|
|||
| |
|
|||
Note 8. IPO related expenses
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Legal fees |
|
|||
|
Consultant fees |
|
|||
|
Audit fees |
|
|||
|
NASDAQ Listing fee |
|
|||
| |
|
|||
F-21
|
Gelteq Limited |
|
Note 9. Depreciation and amortisation expense
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Amortisation expenses |
|
|
||
|
Depreciation on machinery |
|
|||
|
Depreciation expense on right-of-use assets |
|
|||
| | | |||
Note 10. Research expenses
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Product research and development expenses |
|
|
||
Note 11. Employment expenses
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Wages and salaries |
|
|
||
|
Superannuation contribution – employees |
|
|
||
|
Accrued leave expenses |
|
|
||
| |
|
|||
Note 12. Finance costs
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Interest expense on Shareholder loans (refer to note 20) |
|
|
||
|
Amortisation of discount on convertible notes (refer to note 20) |
|
|||
|
Interest on Convertible notes (refer to note 20) |
|
|
||
|
Interest and finance charges – Others |
|
|
||
| |
|
|||
F-22
|
Gelteq Limited |
|
Note 13. Income tax expense/(benefit)
|
Consolidated |
||||||
|
December |
December |
|||||
|
$ |
$ |
|||||
|
Numerical reconciliation of income tax expense/(benefit) and tax at the statutory rate |
|
|
||||
|
Loss before income tax expense |
( |
) |
( |
) |
||
| Tax at the statutory tax rate of |
( |
) |
( |
) |
||
|
Tax effect amounts which are not deductible/(taxable) in calculating taxable income: |
|
|
||||
|
Permanent differences |
|
|
|
|
||
|
Timing differences (not meeting deferred asset criteria) |
( |
) |
( |
) |
||
|
Carry forward losses (not meeting deferred asset criteria) |
|
|
|
|
||
|
Income tax expense/(benefit) |
|
|
||||
Note 14. Cash and cash equivalents
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
Cash at bank |
|
|
||
Note 15. Trade and other receivables
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
GST |
|
|
||
|
Other debtors – research and development tax refund receivable |
|
|
||
|
Accounts receivables |
|
|||
| |
|
|||
Note 16. Prepayments and other assets
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
Prepaid Expenses |
|
|
||
|
Advance for equipment |
|
|
||
|
Prepayment* |
|
|
||
|
Advance payments to vendors for supply of raw materials |
|
|||
|
Other deposits** |
|
|||
| |
|
|||
____________
*
**
F-23
|
Gelteq Limited |
|
Note 17. Intangible assets
|
Consolidated |
||||||
|
December |
June |
|||||
|
$ |
$ |
|||||
|
Non-current assets |
|
|
||||
|
Trade Secrets and Patents – at cost |
|
|
|
|
||
|
Less: Accumulated amortisation |
( |
) |
( |
) |
||
|
Net carrying value |
|
|
|
|
||
|
Patents and trademarks – at cost |
|
|
|
|
||
|
Add: Additions |
|
|
|
|
||
|
Less: Accumulated amortisation |
( |
) |
( |
) |
||
|
Net carrying value |
|
|
|
|
||
| |
|
|
|
|||
Reconciliation
Reconciliations of the written down values at the beginning and end of the current and previous financial period are set out below:
|
Consolidated |
Trade |
Patents & |
Total |
||||||
|
$ |
$ |
$ |
|||||||
|
Balance at 1 July 2023 |
|
|
|
|
|
|
|||
|
Additions |
|
|
|
|
|
||||
|
Amortisation expense |
( |
) |
( |
) |
( |
) |
|||
|
Balance at June 2024 |
|
|
|
|
|
|
|||
|
Additions |
|
|
|
|
|
||||
|
Amortisation expense |
( |
) |
( |
) |
( |
) |
|||
|
Balance at 31 December 2024 |
|
|
|
|
|
|
|||
Trade secrets were acquired during 2021 financial year by the Consolidated Entity and are amortised over its useful life estimate of
Assessment for impairment — 31 December 2024
Methodology
An impairment loss expense in the profit or loss is recognised when the carrying amount of an asset exceeds its recoverable amount. The Consolidated Entity determined the recoverable amounts of the Gelteq Consolidated Entity as one CGU using a value in use approach
The recoverable amount of the CGU has been determined by a forecast model that estimated the future cash flows based on budgets and forecasts for years prepared by management. As part of a valuation of the intangible assets by an independent expert valuer performed as at 30 June 2024, the independent expert valuers extended the forecasts for an additional
F-24
|
Gelteq Limited Note 17. Intangible assets (cont.) |
|
These cash flows were then discounted to their present value using a discount rate that reflects current market assessments of the time value of money and the risks specific to the CGU. Management then cross-checked the total of the discounted cash flows against the trading of the Company’s shares.
A reference to Financial Years (FY), refers to a period covering July 1st to June 30th the next year. A reference to a calendar year (CY) refers to the period from January 1st to December 31st of the same year.
The discounted cash flow model used in the assessment of fair value less cost to sell is sensitive to a number of key assumptions, including revenue growth rates, discount rates and operating costs. These assumptions can change over short periods of time and can have a significant impact on the carrying value of the assets. For any AUD figures presented from the valuation analysis, these have been obtained by conversion from USD at an exchange rate of
Fair value less cost to sell and key assumptions
The Company estimates the fair value less cost to sell of the Gelteq Consolidated Entity cash generating unit (CGU) using discounted cash flows. Management assumptions were developed incorporating internal and external market information, although the extent to which they rely on past experience of the Consolidated Entity is limited given the consolidated entity has not yet started full scale operations, pending completion of preparatory activities where necessary, with external sources of information having been adjusted to reflect factors specific to the Consolidated Entity. Fair value less cost to sell is categorised within level 3 of the fair value hierarchy.
For the reporting period ended 31 December 2024, the recoverable amount of the CGU was determined based on fair value less cost to sell calculations which required the use of key assumptions:
Operating Segments
The Consolidated Entity’s cash flows are generated from one CGU which covers nutraceuticals for humans and animals, pharmaceutical for humans and animals and controlled substances.
Cash Flow projections
• The calculations used cash flow projections based on financial budgets and forecasts approved by management covering CY25 to CY29. The projections included negative undiscounted operating cash flows between CY25 and CY26 before making positive operating returns from CY27 onwards as the business scales up operations and operating margins that are in line with industry averages in similar industries. A full
• A pre-tax discount rate range of
F-25
|
Gelteq Limited Note 17. Intangible assets (cont.) |
|
Revenue —
• Management have implemented a hybrid revenue model with revenue generated from manufacturing and royalties (on each individual order).
• The forecast model is based on a
Gross Margins
• Gross margin is forecast to increase from
Operating Expense
• The largest operating expense is employee costs. Salary and benefits are forecast to increase by
EBITDA
• The forecast model is based on a long-term EBITDA margin of
CAPEX
• No material Capex has been forecast as the costs borne by Gelteq in working with clients to develop products is included in other forecast expenses. As such, forecast capex for relevant supporting assets is $
Amortisation
• Amortisation has been estimated at
Tax Rate
• A tax rate of
F-26
|
Gelteq Limited Note 17. Intangible assets (cont.) |
|
Working Capital
• Model forecasts the receivables at 30 days and payables at 31 days in line with management expectations. Payables days are only applied to operating expenses as all manufacturing costs are paid prior to dispatch to customers.
Other balance Sheet Items
• There are no other assumptions that result in material balance sheet movements that affect relevant forecast cash flow.
Terminal growth rate
• Long term growth rate, used for the terminal value calculation, is
Apart from the considerations described in determining the value-in-use of the cash-generating units described above, management is not currently aware of any other probable changes that would necessitate changes in its key estimates
Impairment
The Consolidated Entity has performed an impairment assessment based on its cash generating unit (CGU).
The Consolidated Entity determined that the recoverable amount in relation the CGU exceeded its carrying value of assets as at 31 December 2024, therefore no adjustment to its carrying value (impairment) was required.
The directors have reviewed and are comfortable with the significant assumptions determined by management. Based on the above, the directors believe that no impairment charge is required to the value of the intangible asset at 31 December 2024
Sensitivity
The sensitivities on the updated discounted cash flow model are as follows:
• Revenue would require a reduction of
• EBITDA margin would need a reduction of
• The discount rate would be required to increase to
• Long Term growth rate would need to be reduced to be in negative (consistent with the 30 June, 2024, valuation) in the cashflow modelling before the intangible asset value would need to be impaired, with all other assumptions remaining constant.
Management believes that other reasonable changes in the key assumptions on which the recoverable amount on which the intangible asset is based would not cause the carrying amount to exceed its recoverable amount.
Management notes that if performance is not as expected, an impairment charge against these assets could be recognised in the next financial year’s accounts. This estimation of uncertainty is expected to reduce over time as the Consolidated Entity’s business develops and matures.
F-27
|
Gelteq Limited |
|
Note 18. Trade and other payables
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
Current liabilities |
||||
|
Trade payables |
|
|
||
|
Accruals |
|
|
||
|
Payroll tax payable |
|
|
||
|
Wages Payable |
|
|
||
|
PAYG Withholding Payable |
|
|
||
|
Superannuation Payable |
|
|
||
|
Insurance Funding |
|
|
||
| |
|
|||
Due to their short-term nature, the directors consider that the carrying amount of trade payables approximates to their fair value. No interest is payable on amounts classified as trade and other payables.
Note 19. Deferred revenue
|
Consolidated |
|||||
|
December |
June |
||||
|
$ |
$ |
||||
|
Current liabilities |
|
||||
|
Deferred Revenue |
|
|
|
||
|
Reconciliation |
|
||||
|
Reconciliation of the written down values at the beginning and end of the current and previous financial period are set out below: |
|
||||
|
Opening balance |
|
|
|
||
|
Payments received in advance |
|
|
|||
|
Transfer to revenue – amount forgiven |
( |
) |
|||
|
Closing balance |
|
|
|
||
Unsatisfied performance obligations
The aggregate amount of the transaction price allocated to the performance obligations that are unsatisfied at the end of the reporting period was $
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
12 to 18 months |
|
|
||
F-28
|
Gelteq Limited |
|
Note 20. Borrowings
| Consolidated | |||||
| December | June | ||||
| $ | $ | ||||
| Current liabilities |
| ||||
| Loan – Director(i) | |
| | ||
| Loan from associated entities(ii) | |
| | ||
| Shareholder Loans(iii) | |
| | ||
| Convertible notes payable(iv) | |
| |||
| Debt discount(v) | ( | ) | |||
| |
| | |||
| Non-current liabilities |
| ||||
| Loan from Director (term – | |
| | ||
| Convertible notes payable(iv) |
| | |||
| |
| | |||
| |
| | |||
Loans from Directors
(i)
Loan from associated entities
(ii)
Shareholder loans
(iii)
As part of the loan agreement, the Company issued
The Company has recognised the shareholders loans initially at fair value of $
On 3 January 2023, the shareholders loans were extended for an additional 12 months at an interest rate of
Subsequent to 30 June 2024, the Company and the lending shareholders agreed to extend the loan maturity until 31 December 2025.
F-29
|
Gelteq Limited Note 20. Borrowings (cont.) |
|
The table below shows the movement of Shareholder loans during the respective periods.
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
Opening Shareholder Loan balance |
|
|
||
|
Interest accrued during the year* |
|
|
||
| |
|
|||
____________
*
Convertible notes
(iv)
• in the case of a Listing, the price per Share set for the underlying securities that are offered for issue as part of the Listing;
• in the case of a Sale Event, the price per Share set for the underlying securities that are to be sold as part of the Sale Event; and
• in the case of a Qualifying Transaction, the price per Share set for the underlying securities that are to be issued as part of the Qualifying Transaction
• of which the Noteholder has a conversion discount of
The convertible note balance as at 31 December 2024 comprises of convertible note funds received $
Since the year ended June 30, 2023, the Company has issued the following additional convertible notes (on the same terms and conditions as the previous convertible notes);
• September 2023, $
• October 2023, $
The total amount raised from the convertible note issue was $
On 2 February 2024, the Board of Directors approved the issuance of convertible notes (the “February 2024 Convertible Note”) to raise up to AUD$
On 27 May 2024, the Board of Directors approved the issuance of convertible notes (the “May 2024 Convertible Note”) to raise up to AUD$
F-30
|
Gelteq Limited Note 20. Borrowings (cont.) |
|
Each holder of Convertible Note may, on a Liquidity event, or at least 90 days prior to Maturity, may elect to either Convert their Notes or redeem for Australian cash repayment. If the Noteholder elects to Convert, the number of fully paid ordinary shares to be issued in satisfaction of the Convertible Notes will be determined by the market value being, determined as;
• in the case of a Listing, the price per Share set for the underlying securities that are offered for issue as part of the Listing;
• in the case of a Sale Event, the price per Share set for the underlying securities that are to be sold as part of the Sale Event; and
• in the case of a Qualifying Transaction, the price per Share set for the underlying securities that are to be issued as part of the Qualifying Transaction of which the Noteholder has a conversion discount of
The table below shows the movement of Convertible Notes during the respective periods.
|
Consolidated |
|||||
|
December |
June |
||||
|
$ |
$ |
||||
|
Opening convertible note balance |
|
|
|
||
|
Convertible notes issued – received in cash |
|
|
|
||
|
Convertible notes issued – accrued (owing) |
( |
) |
|||
|
Interest accrued |
|
|
|
||
| |
|
|
|||
The table below shows the movement of Debt discount on Convertible Notes during the respective periods.
|
Consolidated |
|||||
|
December |
June |
||||
|
$ |
$ |
||||
|
Opening convertible note Debt discount balance |
|
||||
|
Debt discount on convertible notes recognised during the period |
|
|
|||
|
Amortisation of discount on convertible notes during the period |
( |
) |
|||
| |
|
||||
There was repayment of interest or loans/convertible notes during the period ended 31 December 2024 (30 June 2024: ).
Embedded derivative on convertible notes
(v)
The embedded derivative was valued using a Black-Scholes valuation model as at the Company’s IPO date with following key assumptions:
• Company stock price on measurement date: $
• Risk free rate:
F-31
|
Gelteq Limited Note 20. Borrowings (cont.) |
|
• Term:
• Volatility:
This calculation produced an estimated fair value of the embedded derivative of $
Note 21. Issued capital
|
Consolidated |
||||||||
|
December |
June |
December |
June |
|||||
|
Shares |
Shares |
$ |
$ |
|||||
|
Ordinary shares – fully paid |
|
|
|
|
||||
Movements in ordinary share capital
|
Details |
Date |
Shares |
Issue Price |
$ |
||||||
|
Opening balance |
1 July 2024 |
|
$ |
|
|
|
||||
|
Initial public offering |
30 October 2024 |
|
$ |
|
|
|
||||
|
Shares issued in lieu of broker fees |
31 October 2024 |
|
$ |
|
|
|||||
|
Shares issued in Lieu of Marketing fees |
15 November 2024 |
|
$ |
|
|
|
||||
|
Shares issued in Lieu of Investor fees |
25 November 2024 |
|
$ |
|
|
|
||||
|
Share issued in lieu of advisory fee |
31 October 2024 |
|
$ |
|
|
|
||||
|
Capital raising cost |
$ |
|
( |
) |
||||||
|
Total |
|
|
|
|
||||||
Ordinary shares
Ordinary shares entitle the holder to participate in dividends and the proceeds on the winding up of the Consolidated Entity in proportion to the number of and amounts paid on the shares held. The fully paid ordinary shares have no par value and the Consolidated Entity does not have a limited amount of authorised capital.
On a show of hands every member present at a meeting in person or by proxy shall have
Capital risk management
The consolidated entity’s objectives when managing capital is to safeguard its ability to continue as a going concern, so that it can provide returns for shareholders and benefits for other stakeholders and to maintain an optimum capital structure to reduce the cost of capital.
F-32
|
Gelteq Limited Note 21. Issued capital (cont.) |
|
Capital is regarded as total equity, as recognised in the statement of financial position, plus net debt. Net debt is calculated as total borrowings less cash and cash equivalents. The Consolidated Entity may issue shares to investors and suppliers (and employees) time to time to raise capital and compensate for services received.
In order to maintain or adjust the capital structure, the Consolidated Entity may adjust the amount of dividends paid to shareholders, return capital to shareholders, issue new shares or sell assets to reduce debt.
As of December 31, 2024, the company has
Note 22. Dividends
There were dividends paid, recommended or declared during the current or previous financial period.
Note 23. Key management personnel
Key management personnel (KMP) are those persons having authority and responsibility for planning, directing and controlling the activities of the Consolidated Entity, are comprised of the directors of the Company.
Directors
The following persons were directors of Gelteq Limited during the financial period:
|
Mr. Simon Hayden Szewach |
(Executive Chairman) |
|
|
Mr. Nathan Jacob Givoni |
(Executive Director) |
|
|
Mr. Jeffrey W. Olyniec |
(Non-Executive Director) |
|
|
Mr. Philip Dalidakis |
(Non-Executive Director) |
|
|
Prof David Morton |
(Non-Executive Director) |
The aggregate compensation paid/payable to members of key management personnel of the consolidated entity is set out below:
|
Consolidated |
||||
|
December |
December |
|||
|
$ |
$ |
|||
|
Short-term employee benefits |
|
|
||
|
Post-employment benefits |
|
|
||
| | | |||
Note 24. Contingent assets & Liabilities and Commitments
There were contingent liabilities or assets as at 31 December 2024 and 31 December 2023 and no other material commitments as at 31 December 2024 and 31 December 2023.
Note 25. Capital commitments — Property, plant and equipment
The Consolidated Entity had capital commitments for property, plant and equipment as at 31 December 2024 and 30 June 2024.
F-33
|
Gelteq Limited |
|
Note 26. Related party transactions
Parent entity
Gelteq Limited is the parent entity.
Subsidiaries
Key management personnel
Disclosures relating to key management personnel are set out in note 23.
Transactions with related parties
The following transactions occurred with related parties:
|
Consolidated |
||||
|
December |
30 June |
|||
|
$ |
$ |
|||
|
Payment for other expenses: |
||||
|
Interest expense on loans from directors (as part of shareholder loan issue)* |
|
|
||
|
Interest paid to commonly controlled entity* |
|
|
||
|
Management and consulting services** |
|
|
||
____________
*
**
Outstanding balances arising from transactions with related parties:
|
Consolidated |
Consolidated |
|||
|
Receivables from related parties |
December |
30 June |
||
|
$ |
$ |
|||
|
Prepayment* |
|
|
||
|
Accounts receivables** |
|
|||
| |
|
|
Consolidated |
||||
|
Payables to related parties |
December |
June |
||
|
$ |
$ |
|||
|
Payables to by key management personnel directly*** |
|
|||
____________
*
**
***
F-34
|
Gelteq Limited Note 26. Related party transactions (cont.) |
|
Loans to/from related parties
The following balances are outstanding at the reporting date in relation to loans with related parties:
|
Consolidated |
||||
|
Loans from related parties |
December |
June |
||
|
$ |
$ |
|||
|
Beginning of the period |
|
|
||
|
Reclassify >5% holder loan as related party loan (i) |
— |
|
||
|
Interest accrued during the year |
|
|
||
|
Closing Balance |
|
|
||
The Loans from directors relates to loans provided in the year ended 30 June 2022, by Jeffrey Olyniec, Executive Director and B&M Givoni Ltd. a close family member of Nathan Givoni, Executive director of the Company. These loan agreements are compound financial instruments with both debt and equity components. The loans include an equity component of $
The Consolidated Entity has recognised the shareholders loans initially at fair value of $
Subsequent to 30 June, 2024, the loans were extended with a new maturity date of
(i)
|
Consolidated |
||||
|
Loans from associated entities |
December |
30 June |
||
|
$ |
$ |
|||
|
Opening balance |
|
|
||
|
Interest charged |
|
|
||
| |
|
|||
F-35
|
Gelteq Limited Note 26. Related party transactions (cont.) |
|
Convertible notes from Related Parties
|
Consolidated |
||||
|
31 December |
30 June |
|||
|
Opening Balance |
|
|
||
|
Reclassify >5% holder convertible note as related party loan(i) |
|
|||
|
Proceeds from convertible note issue |
|
|
||
|
Interest accrued |
|
|
||
|
Closing Balance |
|
|
||
____________
* The Convertible Notes from directors relates to:
– for 2024, convertible notes received from an entity related to Nathan Givoni, Executive Director, and Jeffrey Olyniec, Non-Executive Director.
– For 2025, convertible notes received from an entity related to Nathan Givoni, Executive Director.
(i)
Terms and conditions
Transactions with related parties have not undergone a formal benchmarking process to establish whether arrangements are conducted under normal market terms and conditions, accordingly, such transactions may not be considered at arm’s length. Related party loans are either unsecured, interest-free and payable on demand or are subject to unsecured loan agreements with fixed terms and interest payable.
Interest-free loans are noted accordingly.
No adjustment has been made to their carrying value. The parent company has not provided any guarantees in relation to any debts incurred by its subsidiaries.
Note 27. Events after the reporting period
No matter or circumstance has arisen since 31 December 2024 that has significantly affected, or may significantly affect the consolidated entity’s operations, the results of those operations, or the consolidated entity’s state of affairs in future financial years.
Note 28. Additional cash flows information
Financing and operating activities not involving cash:
|
Consolidated |
||||
|
December |
June |
|||
|
$ |
$ |
|||
|
Shares issued in lieu of broker fees |
|
|||
|
Shares issued in Lieu of Marketing fees |
|
|||
|
Shares issued in Lieu of Investor fees |
|
|||
|
Share issued in lieu of advisory fee |
|
|||
| |
||||
F-36
|
Gelteq Limited |
|
Note 29. Earnings per share
|
Consolidated |
||||||
|
December |
December |
|||||
|
$ |
$ |
|||||
|
Loss after income tax attributable to the owners of Gelteq Limited |
( |
) |
( |
) |
||
|
Number |
Number |
|||
|
Weighted average number of ordinary shares used in calculating basic earnings per share |
|
|
||
|
Weighted average number of ordinary shares used in calculating diluted earnings per share* |
|
|
|
$ |
$ |
|||||
|
Basic loss per share |
( |
) |
( |
) |
||
|
Diluted loss per share |
( |
) |
( |
) |
||
F-37
|
Gelteq Limited |
|
In accordance with a resolution of the directors of Gelteq Ltd, the directors of the Company declare that:
In the directors’ opinion:
• the financial statements and notes set out in this document are in accordance with requirements of the International Financial Reporting Standards (IFRS), including:
(i) complying with International Accounting Standard IAS 34 — Interim Financial Reporting as issued by the International Accounting Standards Board, and
(ii) present fairly in all material respects the Consolidated Entity’s financial position as at 31 December 2024 and 30 June 2024, and the results of its operations and its cash flows for each of the six month periods ended 31 December 2024 and 31 December 2023, and
• there are reasonable grounds to believe that the Consolidated Entity will be able to pay its debts as and when they become due and payable.
|
On behalf of the directors |
||||
|
/s/ Simon H. Szewach |
||||
|
Simon H. Szewach |
||||
|
Chairman |
||||
|
30 June 2025 |
F-38
|
Gelteq Limited |
|

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of Gelteq Limited
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statement of financial position of Gelteq Limited and its subsidiaries (the Company) as of June 30, 2024, and the related consolidated statements of profit or loss and other comprehensive income, changes in equity, and cash flows for the year ended June 30, 2024, and the related notes (collectively referred to as the “financial statements”). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of June 30, 2024, and the results of its operations and its cash flows for the year ended June 30, 2024, in conformity with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board. The consolidated financial statements of Gelteq Limited as of June 30, 2023 were audited by other auditors whose report dated December 4, 2023 expressed an unqualified opinion on those statements.
Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 4 to the consolidated financial statements, the Company has an excess of current liabilities over current assets and suffered net loss from operations, which raises substantial doubt about its ability to continue as a going concern. Management’s plans regarding those matters are discussed in Note 4. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audit also included evaluating the accounting principles used and the significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe our audit provides a reasonable basis for our opinion.
F-39
|
Gelteq Limited |
|
Critical Audit Matter
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that were communicated, or required to be communicated, to the audit committee and that: (1) relate to accounts or disclosures that are material to the consolidated financial statements and (2) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing separate opinion on the critical audit matter or on the accounts or disclosures to which they relate.
Intangible Assets Impairment Consideration
As discussed in Note 20 to the consolidated financial statements, the Company evaluates intangible assets for impairment annually in accordance with IAS 36 and recognizes an impairment loss when the carrying amount of an asset exceeds its recoverable amount. The Company evaluates the impairment on a cash-generating unit basis which it has determined to be the Gelteq consolidated entity. The recoverable amount of the cash-generating unit has been determined by a forecast model that estimates the future cash flows based on budgets and forecasts discounted for current market assessments of the time value of money and the risks specific to the cash-generating unit.
Auditing management’s evaluation of projected future cash flows involves significant judgement, given the fact that the Company uses management estimates on future revenues and expenses which are not able to be substantiated.
To evaluate the appropriateness and accuracy of the assessment by management, we performed our own analysis based on the market value of the assets.
We have served as the Company’s auditor since 2024
The Woodlands, TX
November 15, 2024
PCAOB ID #2738
F-40
|
Gelteq Limited |
|

|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
Level 9 | 1 York Street | Sydney | NSW | 2000 |
|
|
To the Board of Directors and Shareholders of Gelteq Limited |
sydney@uhyhnsyd.com.au |
Opinion on the Financial Statements
We have audited the accompanying consolidated statement of financial position of Gelteq Limited and its subsidiaries (together the “Consolidated Entity”) as of June 30, 2023, and the related consolidated statements of profit or loss and other comprehensive income, changes in equity, and cash flows for the year ended June 30, 2023, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Consolidated Entity as of June 30, 2023, and the results of their operations and their cash flows for the year ended June 30, 2023, in conformity with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board.
Substantial Doubt about the Consolidated Entity’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that the Consolidated Entity will continue as a going concern. As discussed in Note 4 to the financial statements, the Consolidated Entity is in a current liability position at June 30, 2023 and has suffered recurring losses from operations. These conditions raise substantial doubt about the Consolidated Entity’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 4 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Consolidated Entity’s management. Our responsibility is to express an opinion on the Consolidated Entity’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Consolidated Entity in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Consolidated Entity is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Consolidated Entity’s internal control over financial reporting. Accordingly, we express no such opinion.
F-41
|
Gelteq Limited |
|
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provide a reasonable basis for our opinion.

UHY Haines Norton
We have served as the Consolidated Entity’s auditor since 2021.
Sydney, Australia
4 December 2023
|
An association of independent firms in Australia and New Zealand and a member of UHY International, a network of independent accounting and consulting firms. |
||
|
UHY Haines Norton — ABN 85 140 758 156 NSWBN 98 133 826 |
|
|
|
Liability limited by a scheme approved under Professional Standards Legislation. |
F-42
|
Gelteq Limited |
|
|
Consolidated |
||||||||
|
Note |
30 June |
30 June |
||||||
|
$ |
$ |
|||||||
|
Revenue |
|
|
||||||
|
Revenue from contracts with customers |
6 |
|
|
|
||||
|
|
|
|||||||
|
Other income |
7 |
|
|
|
|
|||
|
Gains on loan modifications |
23 |
|
|
|
||||
|
|
|
|||||||
|
Expenses |
|
|
||||||
|
Raw materials and consumables expenses |
|
( |
) |
|||||
|
Employment expenses |
8 |
( |
) |
( |
) |
|||
|
Corporate expenses |
9 |
( |
) |
( |
) |
|||
|
IPO related expenses |
10 |
( |
) |
( |
) |
|||
|
Depreciation and amortisation expense |
11 |
( |
) |
( |
) |
|||
|
Research expenses |
12 |
( |
) |
( |
) |
|||
|
Advertising and marketing expense |
( |
) |
( |
) |
||||
|
Legal expense |
|
( |
) |
|||||
|
Consulting fees |
( |
) |
( |
) |
||||
|
Other expenses |
( |
) |
( |
) |
||||
|
Inventory – write off |
( |
) |
|
|||||
|
|
|
|||||||
|
Operating loss |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Finance costs |
13 |
( |
) |
( |
) |
|||
|
|
|
|||||||
|
Loss before income tax expense |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Income tax expense |
14 |
|
|
|||||
|
|
|
|||||||
|
Loss after income tax expense for the year attributable to the owners of Gelteq Limited |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Other comprehensive income for the year, net of tax |
|
|
||||||
|
|
|
|||||||
|
Total comprehensive loss for the year attributable to the owners of Gelteq Limited |
( |
) |
( |
) |
||||
|
$ |
$ |
|||||||
|
Basic loss per share |
39 |
( |
) |
( |
) |
|||
|
Diluted loss per share |
39 |
( |
) |
( |
) |
|||
The above consolidated statement of profit or loss and other comprehensive income should be read in conjunction with the accompanying notes
F-43
|
Gelteq Limited |
|
|
Consolidated |
||||||||
|
Note |
30 June |
30 June |
||||||
|
$ |
$ |
|||||||
|
Assets |
|
|
||||||
|
|
|
|||||||
|
Current assets |
|
|
||||||
|
Cash and cash equivalents |
15 |
|
|
|
|
|||
|
Trade and other receivables |
16 |
|
|
|
|
|||
|
Inventories |
17 |
|
|
|
||||
|
Prepayments and other assets |
19 |
|
|
|
|
|||
|
Total current assets |
|
|
|
|
||||
|
|
|
|||||||
|
Non-current assets |
|
|
||||||
|
Fixed assets |
|
|
|
|||||
|
Right-of-use assets |
18 |
|
|
|
||||
|
Intangible assets |
20 |
|
|
|
|
|||
|
Total non-current assets |
|
|
|
|
||||
|
|
|
|||||||
|
Total assets |
|
|
|
|
||||
|
|
|
|||||||
|
Liabilities |
|
|
||||||
|
|
|
|||||||
|
Current liabilities |
|
|
||||||
|
Trade and other payables |
21 |
|
|
|
|
|||
|
Deferred revenue |
22 |
|
|
|
|
|||
|
Borrowings |
23 |
|
|
|
|
|||
|
Lease liabilities |
24 |
|
|
|
||||
|
Employee benefits provisions |
25 |
|
|
|
|
|||
|
Total current liabilities |
|
|
|
|
||||
|
|
|
|||||||
|
Non-current liabilities |
|
|
||||||
|
Borrowings |
23 |
|
|
|
|
|||
|
Employee benefits provisions |
25 |
|
|
|
||||
|
Total non-current liabilities |
|
|
|
|
||||
|
|
|
|||||||
|
Total liabilities |
|
|
|
|
||||
|
|
|
|||||||
|
Net assets |
|
|
|
|
||||
|
|
|
|||||||
|
Equity |
|
|
||||||
|
Issued capital |
26 |
|
|
|
|
|||
|
Accumulated losses |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Total equity |
|
|
|
|
||||
The above consolidated statement of financial position should be read in conjunction with the accompanying notes
F-44
|
Gelteq Limited |
|
|
Consolidated |
Issued |
Reserve |
Accumulated |
Total |
|||||||
|
$ |
$ |
$ |
$ |
||||||||
|
Balance at 1 July 2022 |
|
|
|
( |
) |
|
|
||||
|
|
|
|
|||||||||
|
Loss after income tax expense for the year |
|
( |
) |
( |
) |
||||||
|
Other comprehensive income for the year, net of tax |
|
|
|
||||||||
|
|
|
|
|||||||||
|
Total comprehensive loss for the year |
|
( |
) |
( |
) |
||||||
|
|
|
|
|||||||||
|
Transactions with owners in their capacity as owners: |
|
|
|
||||||||
|
Share-based reserve |
( |
) |
|
|
|
||||||
|
Share capital subscribed (note 26) |
|
|
|
|
|
||||||
|
|
|
|
|||||||||
|
Balance at 30 June 2023 |
|
|
( |
) |
|
|
|||||
|
Consolidated |
Issued |
Reserve |
Accumulated |
Total |
||||||
|
$ |
$ |
$ |
$ |
|||||||
|
Balance at 1 July 2023 |
|
( |
) |
|
|
|||||
|
|
|
|||||||||
|
Loss after income tax expense for the year |
( |
) |
( |
) |
||||||
|
Other comprehensive income for the year, net of tax |
|
|
||||||||
|
|
|
|||||||||
|
Total comprehensive loss for the year |
( |
) |
( |
) |
||||||
|
|
|
|||||||||
|
Balance at 30 June 2024 |
|
( |
) |
|
|
|||||
The above consolidated statement of changes in equity should be read in conjunction with the accompanying notes
F-45
|
Gelteq Limited |
|
|
Consolidated |
||||||||
|
Note |
30 June |
30 June |
||||||
|
$ |
$ |
|||||||
|
Cash flows from operating activities |
|
|
||||||
|
Receipt from Customers |
|
|
|
|
||||
|
Research and development tax incentives |
|
|
|
|
||||
|
Payments to suppliers and employees (inclusive of GST) |
( |
) |
( |
) |
||||
|
Payments to Suppliers IPO (inclusive of GST) |
|
( |
) |
|||||
|
Interest and other finance costs paid |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Net cash used in operating activities |
37 |
( |
) |
( |
) |
|||
|
|
|
|||||||
|
Cash flows from investing activities |
|
|
||||||
|
Payment towards procurement of fixed assets |
( |
) |
( |
) |
||||
|
Payment towards acquisition of intangibles |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Net cash used in investing activities |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Cash flows from financing activities |
|
|
||||||
|
Proceeds from convertible notes |
|
|
|
|
||||
|
Proceeds from issue of shares |
|
|
|
|||||
|
Capital issue costs |
|
( |
) |
|||||
|
Repayment of lease liabilities |
( |
) |
( |
) |
||||
|
|
|
|||||||
|
Net cash from financing activities |
|
|
|
|
||||
|
|
|
|||||||
|
Net increase/(decrease) in cash and cash equivalents |
( |
) |
|
|
||||
|
Cash and cash equivalents at the beginning of the financial year |
|
|
|
|
||||
|
Effects of exchange rate changes on cash and cash equivalents |
|
|
|
|||||
|
|
|
|||||||
|
Cash and cash equivalents at the end of the financial year |
15 |
|
|
|
|
|||
The above consolidated statement of cash flows should be read in conjunction with the accompanying notes
F-46
|
Gelteq Limited |
|
Note 1. General information
The consolidated financial statements covers Gelteq Limited (“Gelteq” or the “Company”) and its controlled entities (referred to herein as the “Consolidated Entity”). Gelteq is a Company limited by shares, incorporated and domiciled in Australia.
The principal activities of the Consolidated Entity during the financial years ended 30 June 2024 and 2023 were the development and testing of a gel based delivery system for humans and animals.
The names of the directors in office at any time during or since the end of the year ended 30 June 2024 are:
Simon Szewach (Executive Chairman)
Nathan Jacob Givoni (Executive Director)
Jeff Olyniec (Non-Executive Director)
Philip Dalidakis (Non-Executive Director)
Prof David Morton (Non-Executive Director)
The names of the directors in office at any time during or since the end of the year ended 30 June 2023 are:
Simon Szewach (Executive Chairman)
Nathan Jacob Givoni (Executive Director)
Jeff Olyniec (Non-Executive Director)
Philip Dalidakis (Non-Executive Director)
Prof David Morton (Non-Executive Director) — Appointed 28 February, 2023
Paul Wynne (Non-Executive Director) — Resigned on 28 February, 2023
The directors have been in office since the start of the financial period to the date of this report unless otherwise stated.
The consolidated financial statements for the year ended 30 June 2024 were authorised for issue, in accordance with a resolution of directors, on 15 November 2024. The directors have the power to amend and reissue the consolidated financial statements.
The consolidated financial statements for the year ended 30 June 2023 were authorised for issue, in accordance with a resolution of directors, on 4 December 2023. The directors have the power to amend and reissue the consolidated financial statements.
Note 2. Basis of preparation
The principal accounting policies adopted in the preparation of the consolidated financial statements are set out in note 3. The policies have been consistently applied to all the years presented, unless otherwise stated.
The consolidated financial statements are presented in Australian Dollars, which is also the Consolidated Entity’s functional currency. Amounts are rounded to the nearest dollar, unless otherwise stated.
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards and International Accounting Standards as issued by the International Accounting Standards Board (IASB) and Interpretations (collectively IFRSs).
The preparation of consolidated financial statements in compliance with adopted IFRS requires the use of certain critical accounting estimates. It also requires the Consolidated Entity’s management to exercise judgment in applying the Consolidated Entity’s accounting policies. The areas where significant judgments and estimates have been made in preparing the consolidated financial statements and their effect are disclosed in note 4.
F-47
|
Gelteq Limited Note 2. Basis of preparation (cont.) |
|
Basis of measurement
The consolidated financial statements have been prepared on a historical cost basis.
The principal accounting policies adopted are consistent with those of the previous financial year and corresponding interim reporting period, unless otherwise stated.
New or amended Accounting Standards and Interpretations adopted — 2024
The Consolidated Entity has adopted all of the new or amended Accounting Standards and Interpretations issued by the International Accounting Standards Board (IASB) that are mandatory for the current reporting period. The adoption of these Accounting Standards and Interpretations did not have any material impact on the financial performance or position of the Consolidated Entity.
The adoption of these Accounting Standards and Interpretations did not have any material impact on the financial performance or position of the Consolidated Entity. The following Accounting Standards and Interpretations are most relevant to the Consolidated Entity:
• IAS 1 and IFRS Practice Statement 2, to require entities to disclose their material accounting policy information rather than their significant accounting policies;
• IAS 7, to clarify that information about measurement bases for financial instruments is expected to be material to an entity’s financial statements;
• IAS 8, to clarify how entities should distinguish changes in accounting policies and changes in accounting estimates;
Any new or amended Accounting Standards or Interpretations that are not yet mandatory have not been early adopted.
New or amended Accounting Standards and Interpretations adopted — 2023
The Consolidated Entity has adopted all of the new or amended Accounting Standards and Interpretations issued by the International Accounting Standards Board (IASB) that are mandatory for the current reporting period.
New Accounting Standards and Interpretations not yet mandatory or early adopted — 2024
Australian Accounting Standards and Interpretations that have recently been issued or amended but are not yet mandatory, have not been early adopted by the Consolidated Entity for the annual reporting period ended 30 June 2024. The Consolidated Entity has not yet assessed the impact of these new or amended Accounting Standards and Interpretations.
IFRS18 Presentation and Disclosure in Financial Statements
This standard is applicable to annual reporting periods beginning on or after 1 January 2027. The standard replaces IAS 1 Presentation of Financial Statements, with many of the original disclosure requirements retained and there will be no impact on the recognition and measurement of items in the financial statements. But the standard will affect presentation and disclosure in the financial statements, including introducing five categories in the statement of profit or loss and other comprehensive income: operating, investing, financing, income taxes and discontinued operations. The standard introduces two mandatory sub-totals in the statement: ‘Operating profit’ and ‘Profit before financing and income taxes’. There are also new disclosure requirements for ‘management-defined performance measures’, such as earnings before interest, taxes, depreciation and amortisation (‘EBITDA’) or ‘adjusted profit’. The standard provides enhanced guidance on grouping of information (aggregation and disaggregation), including whether to present this information in the primary financial statements or in the notes. The Consolidated Entity will adopt this standard
F-48
|
Gelteq Limited Note 2. Basis of preparation (cont.) |
|
from 1 July 2027. As at reporting date, the Consolidated Entity has not completed an assessment on the impact of the standard, but it is expected that there will be a material change to the layout of the statement of profit or loss and other comprehensive income.
Amendments to IAS 1 — Classifications of Liabilities as Current or Non-Current
The amendments are applicable to annual reporting periods beginning on or after 1 January 2024 and early adoption is permitted. This Standard amends IAS 1 to clarify requirements for the presentation of liabilities in the statement of financial position as current or non-current. For example, the amendments clarify that a liability is classified as non-current if an entity has the right at the end of the reporting period to defer settlement of the liability for at least 12 months after the reporting period. The meaning of settlement of a liability is also clarified. The Consolidated Entity will adopt this standard from its application date and where appropriate incorporate the additional disclosures required.
Amendments to IAS 1 — Non-current Liabilities with Covenants
The amendments are applicable to annual reporting periods beginning on or after 1 January 2024 and early adoption is permitted. Amendments specifies that only covenants with which an entity must comply on or before the reporting date affect the classification of a liability as current or non-current. Covenants with which the Consolidated Entity must comply after the reporting date (i.e. future covenants) do not affect a liability’s classification at that date. However, when non-current liabilities are subject to future covenants, companies will now need to disclose information to help users understand the risk that those liabilities could become repayable within 12 months after the reporting date. The Consolidated Entity will adopt this standard from its application date and where appropriate incorporate the additional disclosures required.
Amendments to IFRS 16 — Lease Liability in a Sale and Leaseback
The amendments are applicable to annual reporting periods beginning on or after 1 January 2024 and early adoption is permitted. The Standard amends IFRS 16 Leases to add subsequent measurement requirements for sale and leaseback transactions that satisfy the requirements in IFRS 15 Revenue from Contracts with Customers to be accounted for as a sale.
IFRS 16 already requires a seller-lessee to recognise only the amount of any gain or loss that relates to the rights transferred to the buyer-lessor. The amendments ensure that a similar approach is applied by also requiring a seller-lessee to subsequently measure lease liabilities arising from a leaseback in a way that does not recognise any amount of the gain or loss related to the right of use it retains. The Consolidated Entity will adopt this standard from its application date and where appropriate incorporate the additional disclosures required.
Amendments to IAS 7 and IFRS 7 — Supplier Finance Arrangements
The amendments are applicable to annual reporting periods beginning on or after 1 January 2024. Amendments requires the disclosure of information about an entity’s supplier finance arrangements (also known as supply chain finance, payables finance or reverse factoring arrangements). The new disclosures are designed to enable users of financial statements to assess the effects of those arrangements on the entity’s liabilities and cash flows. The amendments require an entity to disclose the terms and conditions of the arrangements, the carrying amount of the liabilities that are part of the arrangements, the carrying amounts of those liabilities for which the suppliers have already received payment from the finance providers, the range of payment due dates and the effect of non-cash changes. At this time, the application of the amendments is not expected to have a material impact on the Consolidated Entity. The Consolidated Entity will adopt this standard from its application date and where appropriate incorporate the additional disclosures required.
F-49
|
Gelteq Limited Note 2. Basis of preparation (cont.) |
|
Amendments to IAS 21 — Lack of Exchangeability
The amendments are applicable to annual reporting periods beginning on or after 1 January 2025. The Standard amends IAS 21 and IFRS 1 to require entities to apply a consistent approach to determining whether a currency is exchangeable into another currency and the spot exchange rate to use when it is not exchangeable. New disclosures are required to help users assess the impact of using an estimated exchange rate on the financial statements. The Consolidated Entity will adopt this standard from its application date and where appropriate incorporate the additional disclosures required.
New Accounting Standards and Interpretations not yet mandatory or early adopted — 2023
There are a number of standards, amendments to standards, and interpretations which have been issued by the IASB that are effective in future accounting periods that the Consolidated Entity has decided not to adopt early.
The following amendments to standards are applicable to the Company and effective for future reporting periods:
– Disclosure of Accounting Policies (Amendments to IAS 1, IFRS Practice Statement 2 and Amendments to IAS 8), which are effective for accounting periods beginning on or after 1 January 2023;
– Amendment to IFRS 16 — Leases on sale and leaseback, which are effective for accounting periods beginning on or after 1 January 2024;
– Amendment to IAS 1 — Non-current liabilities with covenants which is effective for accounting periods beginning on or after 1 January 2024: and
– Deferred Tax Related to Assets and Liabilities arising from a Single Transaction (Amendments to IAS 12), effective for accounting periods beginning on or after 1 January, 2023.
These standards, which are not yet effective, are not expected to have a material impact on the Consolidated Entity in the current or future reporting periods and on foreseeable future transactions. However, management will continue to assess this closer to the application date of each standard.
Note 3. Summary of significant accounting policies
The principal accounting policies adopted in the preparation of the consolidated financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
(a) Principles of consolidation
The consolidated financial statements incorporate the assets and liabilities of all subsidiaries of Gelteq Limited (‘Company’ or ‘parent entity’) as at 30 June 2024 and 30 June 2023 and the results of all subsidiaries for the years then ended. Gelteq Limited and its subsidiaries together are referred to in these financial statements as the ‘Consolidated Entity’.
Subsidiaries are all those entities over which the Consolidated Entity has control. The Consolidated Entity controls an entity when the Consolidated Entity is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Consolidated Entity. They are de-consolidated from the date that control ceases.
Intercompany transactions, balances and unrealised gains on transactions between entities in the Consolidated Entity are eliminated. Unrealised losses are also eliminated unless the transaction provides evidence of the impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Consolidated Entity.
F-50
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
The acquisition of subsidiaries is accounted for using the acquisition method of accounting. A change in ownership interest, without the loss of control, is accounted for as an equity transaction, where the difference between the consideration transferred and the book value of the share of the non-controlling interest acquired is recognised directly in equity attributable to the parent.
Where the Consolidated Entity loses control over a subsidiary, it derecognises the assets including goodwill, liabilities and non-controlling interest in the subsidiary together with any cumulative translation differences recognised in equity. The Consolidated Entity recognises the fair value of the consideration received and the fair value of any investment retained together with any gain or loss in profit or loss.
(b) Revenue from contracts with customers
Revenue arises mainly from the manufacturing and sale of products. To determine whether to recognise revenue, the consolidated entity follows a 5-step process:
(1) Identifying the contract with a customer
(2) Identifying the performance obligations
(3) Determining the transaction price
(4) Allocating the transaction price to the performance obligations
(5) Recognising revenue when/as the performance obligations are satisfied.
Revenue is recognised either at a point in time or over time, when the consolidated entity satisfies performance obligations by transferring the promised goods or services to its customers.
The consolidated entity recognises contract liabilities for consideration received in respect to unsatisfied performance obligations and reports these amounts as other liabilities (which we refer to as deferred revenues) in the consolidated statement of financial position. Similarly, if the Consolidated Entity satisfies a performance obligation before it receives the consideration, the consolidated entity recognises either a contract asset or a receivable in its consolidated statement of financial position, depending on whether something other than the passage of time is required before the consideration is due.
Sale of Products
Revenue from sale of product for a fixed fee is recognised when or as the consolidated entity transfers control of the assets to the customer.
(c) Research and Development Tax Incentive
The Research and Development Tax Incentive programme provides tax offsets for expenditure on eligible R&D activities. Under the programme, the consolidated entity, is entitled to a refundable R&D credit in Australia on the eligible R&D expenditure incurred on eligible R&D activities. The refundable R&D tax offset is accounted for under IAS 20 Accounting for Government Grants and Disclosure of Government Assistance, as per which the R&D tax offset income is recognised when there is reasonable assurance that it will be received. It is recognised in the consolidated statement of comprehensive income in the same period that the related costs are recognised as expenses and relates to refundable amounts on approved expenses.
(d) Business Combinations/Asset Acquisitions
Business combinations occur where an acquirer obtains control over one or more businesses and results in the consolidation of its assets and liabilities.
F-51
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
A business combination is accounted for by applying the acquisition method, unless it is a combination involving entities or businesses under common control. The business combination will be accounted for from the date that control is obtained, whereby the fair value of the identifiable assets acquired and liabilities (including contingent liabilities) assumed are recognised (subject to certain limited exceptions).
If the acquisition of an asset or a Consolidated Entity of assets does not constitute a business, the individual identifiable assets acquired (including intangible assets) and liabilities are assumed. The cost of the Consolidated Entity shall be allocated to the individual identifiable assets and liabilities on the basis of their relative fair values at the date of purchase. Such a transaction or event does not give rise to goodwill.
Determining whether a particular set of assets and activities is a business should be based on whether the integrated set is capable of being conducted and managed as a business by a market participant. Thus, in evaluating whether a particular set is a business, it is not relevant whether a seller operated the set as a business or whether the acquirer intends to operate the set as a business. In the absence of evidence to the contrary, a particular set of assets and activities in which goodwill is present shall be presumed to be a business. However, a business need not have goodwill.
(e) Income Tax
The income tax expense (income) for the periods ended 30 June 2024 and 2023 comprises current income tax expense (income) and deferred tax expense (income).
Current tax assets and liabilities are offset where a legally enforceable right of set-off exists and it is intended that net settlement or simultaneous realisation and settlement of the respective asset and liability will occur. Deferred tax assets and liabilities are offset where: (a) a legally enforceable right of set-off exists; and (b) the deferred tax assets and liabilities relate to income taxes levied by the same taxation authority on either the same taxable entity or different taxable entities where it is intended that net settlement or simultaneous realisation and settlement of the respective asset and liability will occur in future periods in which significant amounts of deferred tax assets or liabilities are expected to be recovered or settled.
Deferred income tax expense reflects movements in deferred tax asset and deferred tax liability balances during the period, as well as unused tax losses.
Current and deferred income tax expense (income) is charged or credited outside profit or loss when the tax relates to items that are recognised outside profit or loss or arising from a business combination.
Except for business combinations, no deferred income tax is recognised from the initial recognition of an asset or liability where there is no effect on accounting or taxable profit or loss.
A deferred tax liability shall be recognised for all taxable temporary differences, except to the extent that the deferred tax liability arises from:
(a) the initial recognition of goodwill; or
(b) the initial recognition of an asset or liability in a transaction which:
(i) is not a business combination; and
(ii) at the time of the transaction, affects neither accounting profit nor taxable profit (tax loss).
Deferred tax assets and liabilities are calculated at the tax rates that are expected to apply to the period when the asset is realised or the liability is settled and their measurement also reflects the manner in which management expects to recover or settle the carrying amount of the related asset or liability.
Deferred tax assets relating to temporary differences and unused tax losses are recognised only to the extent that it is probable that future taxable profit will be available against which the benefits of the deferred tax asset can be utilised.
F-52
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
(f) Fair Value of Assets and Liabilities
The Consolidated Entity measures some of its assets and liabilities at fair value on either a recurring or non-recurring basis, depending on the requirements of the applicable Accounting Standard.
Fair value is the price the Consolidated Entity would receive to sell an asset or would have to pay to transfer a liability in an orderly (i.e. unforced) transaction between independent, knowledgeable and willing market participants at the measurement date.
As fair value is a market-based measure, the closest equivalent observable market pricing information is used to determine fair value. Adjustments to market values may be made having regard to the characteristics of the specific asset or liability. The fair values of assets and liabilities that are not traded in an active market are determined using one or more valuation techniques. These valuation techniques maximise, to the extent possible, the use of observable market data.
To the extent possible, market information is extracted from either the principal market for the asset or liability (i.e. the market with the greatest volume and level of activity for the asset or liability) or, in the absence of such a market, the most advantageous market available to the entity at the end of the reporting period (i.e. the market that maximises the receipts from the sale of the asset or minimises the payments made to transfer the liability, after taking into account transaction costs and transport costs).
For non-financial assets, the fair value measurement also takes into account a market participant’s ability to use the asset in its highest and best use or to sell it to another market participant that would use the asset in its highest and best use.
The fair value of liabilities and the entity’s own equity instruments (excluding those related to share-based payment arrangements) may be valued, where there is no observable market price in relation to the transfer of such financial instruments, by reference to observable market information where such instruments are held as assets. Where this information is not available, other valuation techniques are adopted and, where significant, are detailed in the respective note to the consolidated financial statements.
(g) Financial Instruments
Initial recognition and measurement
Financial assets and financial liabilities are recognised when the entity becomes a party to the contractual provisions of the instrument. For financial assets, this is equivalent to the date that the Consolidated Entity commits itself to either purchase or sell the asset (i.e. trade date accounting is adopted).
Financial instruments (except for trade receivables) are initially measured at fair value plus transactions costs, except where the instrument is classified ‘at fair value through profit or loss’ in which case transactions costs are recognised as expenses in profit or loss immediately. Where available, quoted prices in an active market are used to determine fair value. In other circumstances, valuation techniques are adopted.
Trade receivables are initially measured at the transaction price if the trade receivables do not contain a significant financing component or if the practical expedient was applied as specified in IFRS 15: Revenue from Contracts with Customers.
F-53
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Classification and subsequent measurement
Financial liabilities
Financial liabilities are subsequently measured at:
– amortised cost; or
– fair value through profit and loss.
A financial liability is measured at fair value through profit and loss if the financial liability is:
– a contingent consideration of an acquirer in a business combination to which IFRS 3: Business Combinations applies;
– held for trading; or
– initially designated as at fair value through profit or loss.
All other financial liabilities are subsequently measured at amortised cost using the effective interest method. The effective interest method is a method of calculating the amortised cost of a debt instrument and of allocating interest expense to profit or loss over the relevant period.
The effective interest rate is the internal rate of return of the financial asset or liability. That is, it is the rate that exactly discounts the estimated future cash flows through the expected life of the instrument to the net carrying amount at initial recognition.
Any gains or losses arising on changes in fair value are recognised in profit or loss to the extent that they are not part of a designated hedging relationship.
The change in fair value of the financial liability attributable to changes in the issuer’s credit risk is taken to other comprehensive income and is not subsequently reclassified to profit or loss. Instead, it is transferred to retained earnings upon derecognition of the financial liability.
If taking the change in credit risk to other comprehensive income enlarges or creates an accounting mismatch, these gains or losses should be taken to profit or loss rather than other comprehensive income. A financial liability cannot be reclassified.
Financial assets
Financial assets are subsequently measured at:
– amortised cost;
– fair value through other comprehensive income; or
– fair value through profit or loss.
Measurement is on the basis of two primary criteria:
– the contractual cash flow characteristics of the financial asset; and
– the business model for managing the financial assets.
A financial asset that meets the following conditions is subsequently measured at amortised cost:
• the financial asset is managed solely to collect contractual cash flows; and
• contractual terms within the financial asset give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding on specified dates.
F-54
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
A financial asset that meets the following conditions is subsequently measured at fair value through other comprehensive income:
• the contractual terms within the financial asset give rise to cash flows that are solely payments of principal and interest on the principal amount outstanding on specified dates; and
• the business model for managing the financial asset comprises both contractual cash flows collection and the selling of the financial asset.
By default, all other financial assets that do not meet the measurement conditions of amortised cost and fair value through other comprehensive income are subsequently measured at fair value through profit or loss.
The Consolidated Entity initially designates a financial instrument as measured at fair value through profit or loss if:
• it eliminates or significantly reduces a measurement or recognition inconsistency (often referred to as an “accounting mismatch”) that would otherwise arise from measuring assets or liabilities or recognising the gains and losses on them on different bases;
• it is in accordance with the documented risk management or investment strategy and information about the groupings is documented appropriately, so the performance of the financial liability that is part of a group of financial liabilities or financial assets can be managed and evaluated consistently on a fair value basis; and
• it is a hybrid contract that contains an embedded derivative that significantly modifies the cash flows otherwise required by the contract.
The initial measurement of financial instruments at fair value through profit or loss is a one-time option on initial classification and is irrevocable until the financial asset is derecognised.
Derecognition
Derecognition of financial liabilities
A liability is derecognised when it is extinguished (i.e. when the obligation in the contract is discharged, cancelled or expires). An exchange of an existing financial liability for a new one with substantially modified terms, or a substantial modification to the terms of a financial liability, is treated as an extinguishment of the existing liability and recognition of a new financial liability.
The difference between the carrying amount of the financial liability derecognised and the consideration paid and payable, including any non-cash assets transferred or liabilities assumed, is recognised in profit or loss.
Derecognition of financial assets
A financial asset is derecognised when the holder’s contractual rights to its cash flows expires, or the asset is transferred in such a way that all the risks and rewards of ownership are substantially transferred.
All the following criteria need to be satisfied for the derecognition of a financial asset:
• the right to receive cash flows from the asset has expired or been transferred;
• all risk and rewards of ownership of the asset have been substantially transferred; and
• the Consolidated Entity no longer controls the asset (i.e. it has no practical ability to make unilateral decisions to sell the asset to a third party).
F-55
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
On derecognition of a financial asset measured at amortised cost, the difference between the asset’s carrying amount and the sum of the consideration received and receivable is recognised in profit or loss.
On derecognition of a debt instrument classified as fair value through other comprehensive income, the cumulative gain or loss previously accumulated in the investment revaluation reserve is reclassified to profit or loss.
(h) Impairment of assets
At the end of each reporting period, the Consolidated Entity assesses whether there is any indication that an asset may be impaired. The assessment will include considering external sources of information and internal sources of information, including dividends received from subsidiaries, associates or joint ventures deemed to be out of pre-acquisition profits. If such an indication exists, an impairment test is carried out on the asset by comparing the recover able amount of the asset, being the higher of the asset’s fair value less costs to sell and value in use to the asset’s carrying amount. Any excess of the asset’s carrying amount over its recoverable amount is recognised immediately in profit or loss, unless the asset is carried at a revalued amount in accordance with another Standard. Any impairment loss of a revalued asset is treated as a revaluation decrease in accordance with that other Standard.
Where it is not possible to estimate the recoverable amount of an individual asset, the Consolidated Entity estimates the recoverable amount of the cash-generating unit to which the asset belongs.
Impairment testing is performed annually for goodwill and intangible assets with indefinite lives.
When an impairment loss subsequently reverses, the carrying amount of the asset (or cash-generating unit) is increased to the revised estimate of its recoverable amount, but so that the increased carrying amount does not exceed the carrying amount that would have been determined had no impairment loss been recognised for the asset (or cash-generating unit) in prior years. A reversal of an impairment loss is recognised immediately in profit or loss, unless the relevant asset is carried at a revalued amount, in which case the reversal of the impairment loss is treated as a revaluation increase.
(i) Inventories
Raw materials, work in progress and finished goods are stated at the lower of cost and net realisable value on a ‘first in first out’ basis. Cost comprises of direct materials and delivery costs, direct labour, import duties and taxes, an appropriate proportion of variable and fixed overhead expenditure based on normal operating capacity. Costs of purchased inventory are determined after deducting rebates and discounts received or receivable.
Raw materials, finished goods and work in progress are stated at the lower of cost and net realisable value. Cost comprises of purchase and delivery costs, net of rebates and discounts received or receivable. Costs are assigned to individual items of inventory on the ‘first in first out’ basis.
Net realisable value is the estimated selling price in the ordinary course of business less the estimated costs of completion and the estimated costs necessary to make the sale.
(j) Right-of-use assets
A right-of-use asset is recognised at the commencement date of a lease. The right-of-use asset is measured at cost, which comprises the initial amount of the lease liability, adjusted for, as applicable, any lease payments made at or before the commencement date net of any lease incentives received, any initial direct costs incurred, and, except where included in the cost of inventories, an estimate of costs expected to be incurred for dismantling and removing the underlying asset, and restoring the site or asset.
F-56
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Right-of-use assets are depreciated on a straight-line basis over the unexpired period of the lease or the estimated useful life of the asset, whichever is the shorter. Where the Consolidated Entity expects to obtain ownership of the leased asset at the end of the lease term, the depreciation is over its estimated useful life. Right-of use assets are subject to impairment or adjusted for any remeasurement of lease liabilities.
The Consolidated Entity has elected not to recognise a right-of-use asset and corresponding lease liability for short-term leases with terms of 12 months or less and leases of low-value assets. Lease payments on these assets are expensed to profit or loss as incurred.
(k) Intangible Assets Other than Goodwill
Trade secrets
Trade secrets with finite useful lives that are acquired separately, including those acquired in a business combination recognised separately from goodwill, are carried at cost less accumulated amortisation and accumulated impairment losses. Amortisation is recognised on a straight-line basis over their estimated useful lives which are disclosed below. The estimated useful life and amortisation method are reviewed at the end of each reporting period, with the effect of any changes in estimate being accounted for on a prospective basis.
Research and development
Expenditure during the research phase of a project is recognised as an expense when incurred.
Under IFRS 138, An intangible asset arising from development (or from the development phase of an internal project) shall be recognised if, and only if, an entity can demonstrate all of the following:
(a) the technical feasibility of completing the intangible asset so that it will be available for use or sale.
(b) its intention to complete the intangible asset and use or sell it.
(c) its ability to use or sell the intangible asset.
(d) how the intangible asset will generate probable future economic benefits. Among other things, the entity can demonstrate the existence of a market for the output of the intangible asset or the intangible asset itself or, if it is to be used internally, the usefulness of the intangible asset.
(e) the availability of adequate technical, financial and other resources to complete the development and to use or sell the intangible asset.
(f) its ability to measure reliably the expenditure attributable to the intangible asset during its development.
Development expenditure that does not meet the criteria for capitalisation above are recognised as an expense as incurred.
Patents & trademarks
Patents and trademarks are measured initially at purchase cost and are amortised on a straight line basis over their estimated useful lives.
The amortisation rates used for each class of intangible asset with a finite useful life are:
| Class of Intangible Asset | Amortisation | |
| Trade Secrets | | |
| Patents and Trademarks | |
F-57
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Foreign Currency Transactions and Balances
(l) Functional and presentation currency
The functional currency of each of the Company’s entities is measured using the currency of the primary economic environment in which that entity operates. The functional currency of Gelteq is AU$ dollars. The consolidated financial statements are presented in Australian dollars.
Transactions and balances
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transaction. Foreign currency monetary items are translated at the period-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined.
Exchange differences arising on the translation of monetary items are recognised in profit or loss, except where deferred in equity as a qualifying cash flow or net investment hedge.
Exchange differences arising on the translation of non-monetary items are recognised directly in other comprehensive income to the extent that the underlying gain or loss is directly recognised in other comprehensive income; otherwise the exchange difference is recognised in profit or loss.
(m) Employee Benefit Provisions
Short-term obligations
Liabilities for accumulating annual leave that are expected to be settled wholly within 12 months after the end of the period in which the employees render the related service are recognised in respect of employees’ services up to the end of the reporting period and are measured at the amounts expected to be paid when the liabilities are settled. The liabilities are presented as current employee benefit obligations in the balance sheet.
Other long-term employee benefit obligations
The liabilities for long service leave are not expected to be settled wholly within 12 months after the end of the period in which the employees render the related service. They are therefore measured as the present value of expected future payments to be made in respect of services provided by employees up to the end of the reporting period using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service.
The obligations are presented as current liabilities in the balance sheet if the entity does not have an unconditional right to defer settlement for at least twelve months after the reporting date, regardless of when the actual settlement is expected to occur.
(n) Cash and Cash Equivalents
Cash and cash equivalents include cash on hand, deposits held at call with banks, other short-term highly liquid investments with original maturities of three months or less, and bank overdrafts.
(o) Government Grants
Government grants received on capital expenditure are generally deducted in arriving at the carrying amount of the asset purchased. Grants for revenue expenditure are recognised as other income by the Consolidated Entity. Where retention of a government grant is dependent on the Consolidated Entity satisfying certain criteria, it is initially recognised as deferred income. When the criteria for retention have been satisfied, the deferred income balance is released to the consolidated statement of comprehensive income or netted against the asset purchased.
F-58
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
(p) Trade and other receivables
Trade and other receivables are recognised at amortised cost, less any allowance for expected credit losses.
(q) Trade and Other Payables
Trade and other payables represent the liabilities for goods and services received by the entity that remain unpaid at the end of the reporting period. The balance is recognised as a current liability with the amounts normally paid within 30 days of recognition of the liability.
Trade and other payables are initially measured their fair value and subsequently measured at amortised cost using the effective interest method.
Accruals are recognised when they can be reasonably estimated and attributed to the relevant financial period. They are assessed for fair value and carried at amortised cost. They are derecognised when a liability for payment is raised as a trade or other payable.
(r) Borrowings
Borrowings are initially recognised at fair value, net of transaction costs incurred. Borrowings are subsequently measured at amortised cost. Any difference between the proceeds (net of transaction costs) and the redemption amount is recognised in profit or loss over the year of the borrowings using the effective interest method.
Borrowings are removed from the balance sheet when the obligation specified in the contract is discharged, cancelled or expired. The difference between the carrying amount of a financial liability that has been extinguished or transferred to another party and the consideration paid, including any non-cash assets transferred or liabilities assumed, is recognised in profit or loss as other income or finance costs.
Borrowings are classified as current liabilities unless the Consolidated Entity has an unconditional right to defer settlement of the liability for at least 12 months after the reporting period.
Borrowing Costs
Borrowing costs directly attributable to the acquisition, construction or production of assets that necessarily take a substantial period of time to prepare for their intended use or sale are added to the cost of those assets, until such time as the assets are substantially ready for their intended use or sale.
All other borrowing costs are recognised in profit or loss in the period in which they are incurred.
(s) Convertible Notes
The component parts of the convertible notes issued by the Consolidated Entity are classified separately as financial liabilities and equity in accordance with the substance of the contractual arrangements and the definitions of a financial liability and an equity instrument. A conversion option that will be settled by the exchange of a fixed amount of cash or another financial asset for a fixed number of the Consolidated Entity’s own equity instruments is an equity instrument.
At the date of issue, the fair value of the liability component is estimated using the prevailing market interest rate for a similar non-convertible instrument. This amount is recorded as a liability on an amortised cost basis using the effective interest method until extinguished upon conversion or at the instrument’s maturity date.
The conversion option classified as equity is determined by deducting the amount of the liability component from the fair value of the compound instrument as a whole. This is recognised and included in equity, net of income tax effects, and is not subsequently remeasured. In addition, the conversion option classified as equity will remain in equity until the conversion option is exercised, in which case, the balance recognised in equity will be transferred to share capital.
F-59
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
Where the conversion option remains unexercised at the maturity date of the convertible note, the balance recognised in equity will be transferred to retained earnings. No gain or loss upon conversion or expiration of the conversion option.
Transaction costs that relate to the issue of the convertible notes are allocated to the liability and equity components in proportion to the allocation of the gross proceeds. Transaction costs relating to the equity component are recognised directly in equity. Transaction costs relating to the liability component are included in carrying amount of the liability component and amortised over the lives of the convertible notes using the effective interest method.
(t) Lease liabilities
A lease liability is recognised at the commencement date of a lease. The lease liability is initially recognised at the present value of the lease payments to be made over the term of the lease, discounted using the interest rate implicit in the lease or, if that rate cannot be readily determined, the Consolidated Entity’s incremental borrowing rate. Lease payments comprise of fixed payments less any lease incentives receivable, variable lease payments that depend on an index or a rate, amounts expected to be paid under residual value guarantees, exercise price of a purchase option when the exercise of the option is reasonably certain to occur, and any anticipated termination penalties. The variable lease payments that do not depend on an index or a rate are expensed in the period in which they are incurred.
Lease liabilities are measured at amortised cost using the effective interest method. The carrying amounts are remeasured if there is a change in the following: future lease payments arising from a change in an index or a rate used; residual guarantee; lease term; certainty of a purchase option and termination penalties. When a lease liability is remeasured, an adjustment is made to the corresponding right-of use asset, or to profit or loss if the carrying amount of the right-of-use asset is fully written down.
(u) Goods and Services Tax (GST)
Revenues, expenses and assets are recognised net of the amount of GST, except where the amount of GST incurred is not recoverable from the Australian Taxation Office (ATO).
Receivables and payables are stated inclusive of the amount of GST receivable or payable. The net amount of GST recoverable from, or payable to, the ATO is included with other receivables or payables in the statement of financial position.
(v) Earnings per Share (EPS)
Basic loss per share
Basic earnings per share is calculated by dividing the profit attributable to equity holders of the Company, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the period, adjusted for bonus elements in ordinary shares issued during the period.
Diluted loss per share
Diluted loss per share adjusts the figures used in the determination of basic loss per share to take into account the after-income tax effect of interest and other financing costs associated with dilutive potential ordinary shares and the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares, unless anti-dilutive.
(w) Operating segments
Operating segments are presented using the ‘management approach’, where the information presented is on the same basis as the internal reports provided to the Chief Operating Decision Makers (‘CODM’). The CODM is responsible for the allocation of resources to operating segments and assessing their performance.
F-60
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
(x) Share-based payments
Equity-settled and cash-settled share-based compensation benefits are provided to employees.
Equity-settled transactions
Equity-settled transactions are awards of shares, or options over shares, that are provided to employees in exchange for the rendering of services. Cash-settled transactions are awards of cash for the exchange of services, where the amount of cash is determined by reference to the share price.
The cost of equity-settled transactions are measured at fair value on grant date. Fair value is generally determined using either the Binomial or Black-Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk free interest rate for the term of the option, together with non-vesting conditions that do not determine whether the Consolidated Entity receives the services that entitle the employees to receive payment. No account is taken of any other vesting conditions.
The cost of equity-settled transactions are recognised as an expense with a corresponding increase in equity over the vesting period. The cumulative charge to profit or loss is calculated based on the grant date fair value of the award, the best estimate of the number of awards that are likely to vest and the expired portion of the vesting period. The amount recognised in profit or loss for the period is the cumulative amount calculated at each reporting date less amounts already recognised in previous periods.
Cash-settled transactions
The cost of cash-settled transactions is initially, and at each reporting date until vested, determined by applying either the Binomial or Black-Scholes option pricing model, taking into consideration the terms and conditions on which the award was granted. The cumulative charge to profit or loss until settlement of the liability is calculated as follows:
• during the vesting period, the liability at each reporting date is the fair value of the award at that date multiplied by the expired portion of the vesting period.
• from the end of the vesting period until settlement of the award, the liability is the full fair value of the liability at the reporting date.
All changes in the liability are recognised in profit or loss. The ultimate cost of cash-settled transactions is the cash paid to settle the liability.
There are no cash settled transactions for financial year 2024 or financial year 2023.
Market conditions are taken into consideration in determining fair value. Therefore, any awards subject to market conditions are considered to vest irrespective of whether or not that market condition has been met, provided all other conditions are satisfied.
If equity-settled awards are modified, as a minimum an expense is recognised as if the modification has not been made. An additional expense is recognised, over the remaining vesting period, for any modification that increases the total fair value of the share-based compensation benefit as at the date of modification.
If the non-vesting condition is within the control of the Consolidated Entity or employee, the failure to satisfy the condition is treated as a cancellation. If the condition is not within the control of the Consolidated Entity or employee and is not satisfied during the vesting period, any remaining expense for the award is recognised over the remaining vesting period, unless the award is forfeited.
F-61
|
Gelteq Limited Note 3. Summary of significant accounting policies (cont.) |
|
If equity-settled awards are cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognised immediately. If a new replacement award is substituted for the cancelled award, the cancelled and new award is treated as if they were a modification.
(y) Comparative Figures
When required by Accounting Standards, comparative figures have been adjusted to conform to changes in presentation for the current financial period.
Where the Consolidated Entity retrospectively applies an accounting policy, makes a retrospective restatement or reclassifies items in its financial statements, a third statement of financial position as at the beginning of the preceding period in addition to the minimum comparative consolidated financial statements is presented
Note 4. Critical accounting judgements, estimates and assumptions
The preparation of the consolidated financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the consolidated financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results. The judgements, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities (refer to the respective notes) within the next financial year are discussed below.
Impacts of Covid-19
Judgement has been exercised in considering the impacts that the Coronavirus (COVID-19) pandemic has had, or may have, on the Consolidated Entity based on known information. This consideration extends to the nature of the products and services offered, customers, supply chain, staffing and geographic regions in which the Consolidated Entity operates. Other than as addressed in specific notes, there does not currently appear to be either any significant impact upon the condensed consolidated financial statements or any significant uncertainties with respect to events or conditions which may impact the Consolidated Entity unfavorably as at the reporting date or subsequently as a result of the Coronavirus (COVID-19) pandemic.
Estimation of useful lives of assets
The Consolidated Entity determines the estimated useful lives and related depreciation and amortisation charges for its property, plant and equipment and finite life intangible assets. The useful lives could change significantly as a result of technical innovations or some other event. The depreciation and amortization charge will increase where the useful lives are less than previously estimated lives, or technically obsolete or non-strategic assets that have been abandoned or sold will be written off or written down.
Intangible assets
The Consolidated Entity tests annually, or more frequently if events or changes in circumstances indicate impairment, whether indefinite life or finite life intangible assets have suffered any impairment, in accordance with the accounting policy stated in note 3. The recoverable amounts of cash-generating units have been determined based on value-in-use calculations. These calculations require the use of assumptions, including estimated discount rates based on the current cost of capital and growth rates of the estimated future cash flows. Refer to note 20 for details.
F-62
|
Gelteq Limited Note 4. Critical accounting judgements, estimates and assumptions (cont.) |
|
Income tax
The Consolidated Entity is subject to income taxes in the jurisdictions in which it operates. Significant judgement is required in determining the provision for income tax. There are many transactions and calculations undertaken during the ordinary course of business for which the ultimate tax determination is uncertain. The Consolidated Entity recognises liabilities for anticipated tax audit issues based on the Consolidated Entity’s current understanding of the tax law. Where the final tax outcome of these matters is different from the carrying amounts, such differences will impact the current and deferred tax provisions in the period in which such determination is made.
Recognition of deferred tax assets
Deferred tax assets are recognised for deductible temporary differences and carried forward losses, only if the Consolidated Entity considers it is probable that future taxable amounts will be available to utilise those temporary differences and losses.
Leases — Incremental borrowing rate
Where the interest rate implicit in a lease cannot be readily determined, an incremental borrowing rate is estimated to discount future lease payments to measure the present value of the lease liability at the lease commencement date. Such a rate is based on what the Consolidated Entity estimates it would have to pay a third party to borrow the funds necessary to obtain an asset of a similar value to the right-of-use asset, with similar terms, security and economic environment.
Employee benefits provision
As discussed in note 3, the liability for employee benefits expected to be settled more than 12 months from the reporting date are recognised and measured at the present value of the estimated future cash flows to be made in respect of all employees at the reporting date. In determining the present value of the liability, estimates of attrition rates and pay increases through promotion and inflation have been taken into account.
Going Concern 2024
The working capital position as at 30 June 2024 of the Consolidated Entity reflected an excess of current liabilities over current assets of $
The above matters give rise to a material uncertainty that may cast significant doubt over the Consolidated Entity’s ability to continue as a going concern. Therefore, the Consolidated Entity may be unable to realise its assets and discharge its liabilities in the normal course of business at the amounts stated in the consolidated financial statements.
Notwithstanding the above matters, the Directors believe that it is reasonably foreseeable that the Consolidated Entity will be able to continue as a going concern and that it is appropriate to adopt the going concern basis in the preparation of the financial report, after considering the following matters:
• The directors have prepared detailed cash flow projections for a period of at least 12 months from the date of signing this consolidated financial report. The Consolidated Entity’s ability to fund its operations is dependent upon management’s plans and execution, which include raising additional capital, if required, through public offerings, obtaining regulatory approvals for its products and generating revenues from these products and having the ability to be able to reduce expenditure accordingly if required, in order to be able to pay its debts as and when they fall due.
F-63
|
Gelteq Limited Note 4. Critical accounting judgements, estimates and assumptions (cont.) |
|
• During the 2023 financial year, the shareholders loans received on February 4, 2022, had their maturity date extended in January 2023, and approximately $
• On May 27, 2024, the Company’s board of directors approved the issuance of convertible notes (each a “May 2024 Convertible Note”) to raise up to AUD$
• Most importantly, the Company completed its IPO on 30 October 2024, issuing
The Consolidated Entity’s consolidated financial statements have therefore been prepared on a going concern basis which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities should the Consolidated Entity be unable to continue as a going concern.
Going Concern — 2023
As at 30 June 2023, the Consolidated Entity’s current liabilities exceeded current assets by $
During the 2023 financial year, the shareholders loans received on February 4, 2022, had their maturity date extended in January 2023, and approximately $
The above matters give rise to a material uncertainty that may cast significant doubt over the Consolidated Entity’s ability to continue as a going concern. Therefore, the Consolidated Entity may be unable to realise its assets and discharge its liabilities in the normal course of business at the amounts stated in the financial report.
The directors have prepared detailed cash flow projections for the period of 12 months from the date of signing this consolidated financial report. The Consolidated Entity’s ability to fund its operations is dependent upon management’s plans and execution, which include raising additional capital, either through the proposed public offering or private equity, or the issue of additional convertible notes, obtaining regulatory approvals for its products and generating revenues from these products and having the ability to be able to reduce expenditure accordingly if required, in order to be able to pay its debts as and when they fall due.
F-64
|
Gelteq Limited Note 4. Critical accounting judgements, estimates and assumptions (cont.) |
|
On May 5, 2023, the board approved by resolution a raising of up to AUD$
The Consolidated Entity’s consolidated financial statements have been prepared on a going concern basis which contemplates the realization of assets and satisfaction of liabilities and commitments in the normal course of business. The consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities should the Consolidated Entity be unable to continue as a going concern.
Note 5. Operating segments
During the current financial period, the Consolidated Entity operated in
IFRS 8 requires operating segments to be identified on the basis of internal reports about the components of the Consolidated Entity that are regularly reviewed by the chief operating decision maker in order to allocate resources to the segment and to assess its performance. In the current year the board reviews the Consolidated Entity as
Note 6. Revenue from contracts with customers
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Sale of products |
|
|||
All revenues are recognized accordance with the policy at the point in time of delivery.
Disaggregation of revenue by geographical location:
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
United States of America |
| |||
|
Total |
|
|||
Note 7. Other income
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Foreign exchange gain |
|
|
||
|
Research & Development – tax incentive |
|
|
||
|
Other income |
|
|
||
F-65
|
Gelteq Limited |
|
Note 8. Employment expenses
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Wages and salaries |
|
|
||
|
Superannuation contribution – employees |
|
|
||
|
Accrued leave expenses |
|
|
||
|
Payroll tax expense |
|
|
||
|
Long service leave expenses |
|
|||
| |
|
|||
Note 9. Corporate expenses
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Accounting expense |
|
|
||
|
Professional fees |
|
|
||
|
Management fees |
|
|
||
|
Audit fees (non-IPO related) |
|
|||
| |
|
|||
Note 10. IPO related expenses
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Legal fees |
|
|
||
|
Consultant fees |
|
|
||
|
Audit fees |
|
|
||
| |
|
|||
Note 11. Depreciation and amortisation expense
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Amortisation expenses |
|
|
||
|
Depreciation on machinery |
|
|||
|
Depreciation expense on right-of-use assets |
|
|
||
| |
|
|||
Note 12. Research expenses
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Product research and development expenses |
|
|
||
F-66
|
Gelteq Limited |
|
Note 13. Finance costs
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Total interest expense on Shareholder loans (refer to note 23) |
|
|
||
|
Total interest on Convertible notes (refer to note 23) |
|
|
||
|
Interest and finance charges paid/payable on lease liabilities |
|
|
||
|
Finance charges paid – others |
|
|
||
| |
|
|||
Note 14. Income tax expense
|
Consolidated |
||||||
|
30 June |
30 June |
|||||
|
$ |
$ |
|||||
|
Numerical reconciliation of income tax expense and tax at the statutory rate |
|
|
||||
|
Loss before income tax expense |
( |
) |
( |
) |
||
|
|
|
|||||
| Tax at the statutory tax rate of |
( |
) |
( |
) |
||
|
|
|
|||||
|
Tax effect amounts which are not deductible/(taxable) in calculating taxable income: |
|
|
||||
|
Permanent differences |
|
|
|
|
||
|
Timing differences (not meeting deferred asset criteria) |
( |
) |
|
|
||
|
Carry forward losses (not meeting deferred asset criteria) |
|
|
|
|
||
|
Income tax expense |
|
|
||||
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Deferred tax assets not recognised |
||||
|
Deferred tax assets not recognised comprises temporary differences attributable to: |
||||
|
– Payables, accrued expenses and provisions |
|
|
||
|
– Deferred revenue |
|
|
||
|
– Other – Expenses deductible in future periods |
|
|
||
|
– Other – Right of use assets |
|
|||
|
Total deferred tax asset attributable to temporary differences not recognised |
|
|
||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
The amount of unused tax losses for which no deferred tax asset is recognised: |
||||
|
– applicable to the company |
|
|
||
|
– applicable to subsidiaries (not consolidated for tax purposes) |
|
|
||
|
Total |
|
|
||
|
Potential tax benefit @ 25% |
|
|
F-67
|
Gelteq Limited Note 14. Income tax expense (cont.) |
|
The above potential tax benefit for tax losses and other deferred tax assets has not been recognised in the statement of financial position as the recovery of this benefit is uncertain. These tax losses can only be utilised in the future if the continuity of ownership test is passed, or failing that, the same business test is passed, and if the Consolidated Entity has taxable income.
Note 15. Cash and cash equivalents
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
Cash on hand |
|
|||
|
Cash at bank |
|
|
||
| |
|
|||
Note 16. Trade and other receivables
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
Accounts receivable – Convertible notes |
|
|||
|
GST |
|
|||
|
Other debtors – research and development tax refund receivable |
|
|
||
|
Accounts receivable |
|
|
||
| | | |||
The consolidated entity has expected credit losses to trade receivables in 2024 or 2023. All receivables are current as at 30 June 2024 and 30 June 2023.
Due to their short term nature, the directors consider that the carrying value of trade and other receivables approximates their fair value.
Note 17. Inventories
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
Raw materials – at cost |
— |
|
||
The board decided to write off the inventory as the shelf life of the inventory had expired as at 30 June 2024.
F-68
|
Gelteq Limited |
|
Note 18. Right-of-use assets
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Non-current assets |
|
||||
|
Right-of-use assets |
|
|
|||
|
Less: Accumulated depreciation |
( |
) |
|||
| |
|
||||
The Consolidated Entity leases a building for its office space under an agreement which expired on 1 November 2023 and hence the balance is as at 30 June 2024 (total rental payable as at 30 June, 2023 was $
Refer note 33 for further information on related party.
Note 19. Prepayments and other assets
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current assets |
||||
|
Marketing and promotion |
|
|
||
|
Advance for equipment |
|
|
||
|
Prepaid expenses |
|
|
||
|
Advance payments to vendors for supply of raw materials |
|
|||
| |
|
|||
Note 20. Intangible assets
|
Consolidated |
||||||
|
30 June |
30 June |
|||||
|
$ |
$ |
|||||
|
Non-current assets |
|
|
||||
|
Trade Secrets and Patents – at cost |
|
|
|
|
||
|
Less: Accumulated amortisation |
( |
) |
( |
) |
||
|
Net carrying value |
|
|
|
|
||
|
Patents and trademarks – at cost |
|
|
|
|
||
|
Add: Additions |
|
|
|
|
||
|
Less: Accumulated amortisation |
( |
) |
( |
) |
||
|
Net carrying value |
|
|
|
|
||
| |
|
|
|
|||
F-69
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
Reconciliation
Reconciliations of the written down values at the beginning and end of the current and previous financial year are set out below:
|
Trade |
Patents & |
Total |
|||||||
|
$ |
$ |
$ |
|||||||
|
Consolidated |
|
|
|
||||||
|
Balance at 1 July 2022 |
|
|
|
|
|
|
|||
|
Additions |
|
|
|
|
|
||||
|
Amortisation expense |
( |
) |
( |
) |
( |
) |
|||
|
|
|
|
|||||||
|
Balance at 30 June 2023 |
|
|
|
|
|
|
|||
|
Additions |
|
|
|
|
|
||||
|
Amortisation expense |
( |
) |
( |
) |
( |
) |
|||
|
Balance at 30 June 2024 |
|
|
|
|
|
|
|||
Trade secrets were acquired during 2021 financial year by the Consolidated Entity and are amortised over its useful life estimate of
Assessment for impairment — 2024
For the year ended 30 June 2024, management has performed an impairment assessment in accordance with IAS 36. As part of this process, management obtained a valuation of the intangible assets by an independent expert valuer.
Methodology
An impairment loss expense in the profit or loss is recognised when the carrying amount of an asset exceeds its recoverable amount. The Consolidated Entity determined the recoverable amounts of the Gelteq Consolidated Entity as one CGU using a fair value less cost to sell approach.
The recoverable amount of the CGU has been determined by a forecast model that estimated the future cash flows based on budgets and forecasts for five years prepared by management. The independent expert valuers extended the forecasts for an additional
These cash flows were then discounted to their present value using a discount rate that reflects current market assessments of the time value of money and the risks specific to the CGU. The independent valuer then cross-checked the total of the discounted cash flows against the Company’s IPO share price and subsequent trading of the Company’s shares.
A reference to Financial Years (FY), refers to a period covering July 1st to June 30th the next year. A reference to a calendar year (CY) refers to the period from January 1st to December 31st of the same year.
F-70
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
The discounted cash flow model used in the assessment of fair value less cost to sell is sensitive to a number of key assumptions, including revenue growth rates, discount rates and operating costs. These assumptions can change over short periods of time and can have a significant impact on the carrying value of the assets. For any AUD figures presented from the valuation analysis, these have been obtained by conversion from USD at an exchange rate of
Fair value less cost to sell and key assumptions
The Company estimates the fair value less cost to sell of the Gelteq Consolidated Entity cash generating unit (CGU) using discounted cash flows. Management assumptions were developed incorporating internal and external market information, although the extent to which they rely on past experience of the Consolidated Entity is limited given the consolidated entity has not yet started full scale operations, pending completion of capital raising activities where necessary, with external sources of information having been adjusted to reflect factors specific to the Consolidated Entity. Fair value less cost to sell is categorised within level 3 of the fair value hierarchy.
For the 2024 reporting period, the recoverable amount of the CGU was determined based on fair value less cost to sell calculations which required the use of key assumptions:
Operating Segments
• The Consolidated Entity’s cash flows are generated from one CGU which covers nutraceuticals for humans and animals, pharmaceutical for humans and animals and controlled substances.
Cash Flow projections
• The calculations used cash flow projections based on financial budgets and forecasts approved by management covering FY25 to FY29. The projections included negative undiscounted operating cash flows between FY25 and FY27 before making positive operating returns from FY28 onwards as the business scales up operations and operating margins that are in line with industry averages in similar industries. A full
• A pre-tax discount rate range of
Revenue
• Management have implemented a hybrid revenue model with revenue generated from manufacturing and royalties (on each individual order).
• The forecast model is based on a
F-71
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
Gross Margins
• Gross margin is forecast to increase from
Operating Expense
• The largest operating expense is employee costs. Salary and benefits are forecast to increase by
EBITDA
• The forecast model is based on a long-term EBITDA margin of
CAPEX
• No material Capex has been forecast as the costs borne by Gelteq in working with clients to develop products is included in other forecast expenses. As such, forecast capex for relevant supporting assets is $
Tax Rate
• A tax rate of
Working Capital
• Model forecasts the receivables at 30 days and payables at 31 days in line with management expectations. Payables days are only applied to operating expenses as all manufacturing costs are paid prior to dispatch to customers.
Other balance Sheet Items
• There are no other assumptions that result it material balance sheet movements that affect forecast cash flow.
Terminal growth rate
• Long term growth rate, used for the terminal value calculation, is
Apart from the considerations described in determining the value-in-use of the cash-generating units described above, management is not currently aware of any other probable changes that would necessitate changes in its key estimates.
F-72
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
Impairment
The Consolidated Entity has performed an impairment assessment based on its cash generating unit (CGU).
The Consolidated Entity determined that the recoverable amount in relation the CGU exceeded its carrying value of assets as at 30 June 2024, therefore no adjustment to its carrying value (impairment) was required.
The directors have reviewed and are comfortable with the significant assumptions determined by management. Based on the above, the directors believe that no impairment charge is required to the value of the intangible asset at 30 June 2024.
Sensitivity
The sensitivities on the updated discounted cash flow model are as follows:
• Revenue would require a reduction of
• EBITDA margin would need a reduction of
• The discount rate would be required to increase to
• Long Term growth rate would need to be reduced to be in negative (consistent with the 30 June, 2023, valuation) in the cashflow modelling before the intangible asset value would need to be impaired, with all other assumptions remaining constant.
Management believes that other reasonable changes in the key assumptions on which the recoverable amount on intangible asset is based would not cause the carrying amount to exceed its recoverable amount.
Management notes that if performance is not as expected, an impairment charge against these assets could be recognised in future financial years’ accounts. This estimation of uncertainty is expected to reduce over time as the Consolidated Entity’s business develops and matures.
Assessment for impairment — 2023
As disclosed in note 3, management has made judgements and estimates in respect of impairment testing of intangible assets. Since 30 June, 2022, the Company issued
F-73
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
there is still significant headroom (over
Based on the above, the directors believe that no impairment charge is required to the value of the intangible asset at 30 June, 2023.
Methodology
An impairment loss expense in the profit or loss is recognised when the carrying amount of an asset exceeds its recoverable amount. The Consolidated Entity determined the recoverable amounts of the Gelteq Consolidated Entity as one CGU using a fair value less cost to sell approach.
The recoverable amount of the CGU has been determined by a forecast model that estimated the future cash flows based on approved budgets extrapolated for five years by management. The independent valuation experts made a number of material changes to the model, including the revenue generation profile, a decrease in gross margin and an increase in operating expenses and capital expenditure, and extending the forecasts for an additional
Management has continued to adopt this extended period for the purposes of the cash flow estimates included in the Fair value less cost to sell recoverable value as at 30 June 2023. This was discounted to their present value using a discount rate that reflects current market assessments of the time value of money and the risks specific to the CGU. When referring to Financial Years (FY), this refers to a period covering July 1st to June 30th the next year. When referring to a calendar year (CY), this is from January 1st to December 31st of the same year.
The discounted cash flow model used in the assessment of fair value less cost to sell is sensitive to a number of key assumptions, including revenue growth rates, discount rates and operating costs. These assumptions can change over short periods of time and can have a significant impact on the carrying value of the assets. For any AUD figures presented from the valuation analysis, these have been obtained by conversion from USD at an exchange rate of
Fair value less cost to sell and key assumptions
The Company estimates the fair value less cost to sell of the Gelteq Consolidated Entity cash generating unit (CGU) using discounted cash flows. Management assumptions were developed in conjunction with its external valuation advisors. Management incorporated internal and external market information developing these assumptions, although the extent to which they rely on past experience of the Consolidated Entity is limited given the consolidated entity has not yet started full scale operations, pending capital raising activities where necessary, with external sources of information having been adjusted to reflect factors specific to the Consolidated Entity. Fair value less cost to sell is categorised within level 3 of the fair value hierarchy.
For the 2023 reporting period, the recoverable amount of the CGU was determined based on fair value less cost to sell calculations which required the use of key assumptions:
• Operating Segments —
• The Consolidated Entity’s cash flows are generated from one CGU which covers nutraceuticals for humans and animals, pharmaceutical for humans and animals and controlled substances.
• Cash Flow projections —
• The calculations used cash flow projections based on financial budgets approved by management covering FY24 to FY27. With extrapolations using growth assumptions utilized up to the end of CY29. The projections included negative undiscounted operating cash flows between CY23 and
F-74
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
CY25 before making positive operating returns from CY26 onwards as the business scales up operations and operating margins that are in line with industry averages in similar industries. A full
• Weighted average cost of capital of
• Revenue —
• Management have implemented a hybrid revenue model with revenue generated from manufacturing and royalties (on each individual order). For simplicity, the DCF model has excluded royalty revenue from the calculations.
• The model is based on a
• Gross Margins —
• The Consolidated Entity has forecast sales on an exclusive and non-exclusive basis. Exclusive sales are for products that can only be sold by one retailer in an agreed territory. Non-exclusive sales mean more than one party can sell the same product in a particular territory. Higher margins are forecast for exclusive sales as the customer gets the benefit of exclusivity. Management has forecast gross margin on exclusive sales of
• Operating Expense —
• The largest operating expense is employee costs. Salary and benefits are forecast to increase by
• EBITDA —
• The model is based on a long-term EBITDA margin of
• CAPEX —
• Model forecast capex on intangibles at A$
• Amortisation —
• Amortisation has been estimated at
F-75
|
Gelteq Limited Note 20. Intangible assets (cont.) |
|
• Tax Rate
• A tax rate of
• Working Capital
• Model forecasts the receivables and payables at 30 days in line with management expectations. Payables days are only applied to operating expenses as all manufacturing costs are paid prior to dispatch to customers.
• Other balance Sheet Items
• There are no other assumptions that result it material balance sheet movements that affect forecast cash flow.
Apart from the considerations described in determining the value-in-use of the cash-generating units described above, management is not currently aware of any other probable changes that would necessitate changes in its key estimates.
Impairment
The Consolidated Entity has performed an impairment assessment based on its cash generating unit (CGU).
The Consolidated Entity determined that the recoverable amount in relation the CGU exceeded its carrying value of assets as at 30 June 2023, therefore no adjustment to its carrying value (impairment) was required.
The directors have reviewed and are comfortable with the significant assumptions determined by management. Based on the above, the directors believe that no impairment charge is required to the value of the intangible asset at 30 June 2023.
Sensitivity
The sensitivities on the updated discounted cash flow model are as follows:
• Revenue would require a reduction of
• EBITDA margin would need a reduction of
• The discount rate would be required to increase to
• Long Term growth rate would need to be reduced to be in negative in the cashflow modelling before the intangible asset value would need to be impaired, with all other assumptions remaining constant.
Management believes that other reasonable changes in the key assumptions on which the recoverable amount on intangible asset is based would not cause the carrying amount to exceed its recoverable amount.
Management notes that if performance is not as expected, an impairment charge against these assets could be recognised in the next financial year’s accounts. This estimation of uncertainty is expected to reduce over time as the Consolidated Entity’s business develops and matures.
F-76
|
Gelteq Limited |
|
Note 21. Trade and other payables
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current liabilities |
||||
|
Trade payables |
|
|
||
|
Accruals |
|
|
||
|
Payroll tax payable |
|
|
||
|
Wages Payable |
|
|
||
|
PAYG Withholding Payable |
|
|
||
|
Superannuation Payable |
|
|
||
|
Insurance Funding |
|
|||
|
GST payable |
|
|||
| |
|
|||
Due to their short term nature, the directors consider that the carrying amount of trade payables approximates to their fair value. No interest is payable on amounts classified as trade and other payables.
Note 22. Deferred revenue
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Current liabilities |
|
||||
|
Deferred Revenue |
|
|
|
||
|
|
|||||
|
Reconciliation |
|
||||
|
Reconciliation of the written down values at the beginning and end of the current and previous financial year are set out below: |
|
||||
|
|
|||||
|
Opening balance |
|
|
|
||
|
Payments received in advance |
|
|
|
||
|
Transfer to revenue – performance obligations satisfied during the year |
( |
) |
|||
|
Closing balance |
|
|
|
||
Unsatisfied performance obligations
The aggregate amount of the transaction price allocated to the performance obligations that are unsatisfied at the end of the reporting period was $
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
6 to 12 months |
|
|
||
F-77
|
Gelteq Limited |
|
Note 23. Borrowings
| Consolidated | ||||
| 30 June | 30 June | |||
| $ | $ | |||
| Current liabilities | ||||
| Loans from Directors (i) | | | ||
| Loan from associated entities (ii) | | |||
| Shareholder Loans (iii) | | |||
| | | |||
| Non-current liabilities | ||||
| Loan from Director (term – | | | ||
| Loan from associated entities (ii) | | |||
| Shareholder loans (iv) | | |||
| Convertible notes payable (v) | | | ||
| | | |||
| | | |||
Loans from Directors
(i)
Loan from associated entities
(ii)
Shareholder loans — 2024
(iii)
As part of the loan agreement, the Company issued
The Company has recognised the shareholders loans initially at fair value of $
On 3 January 2023, the shareholders loans were extended for an additional 12 months at an interest rate of
Subsequent to 30 June 2024, the Company and the lending shareholders agreed to extend the loan maturity until 31 December 2025.
F-78
|
Gelteq Limited Note 23. Borrowings (cont.) |
|
Shareholder loans — 2023
(iv)
These loan agreements are compound financial instruments with both debt and equity components. The loans include an equity component of $
The resulting gain on the modification of the liability is recognised in the profit and loss.
The table below shows the movement of Shareholder loans during the respective periods.
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Opening Shareholder Loan balance |
|
|
|
||
|
Interest accrued prior to modification |
|
|
|||
|
Shareholder Loan balance at date of modification (January 3, 2023) |
|
|
|||
|
Balance derecognised upon modification |
( |
) |
|||
|
New Fair Value Shareholder Loan Balance post modification |
|
|
|||
|
Interest accrued post modification |
|
|
|
||
| |
|
|
|||
Convertible notes
(v)
• in the case of a Listing, the price per Share set for the underlying securities that are offered for issue as part of the Listing;
• in the case of a Sale Event, the price per Share set for the underlying securities that are to be sold as part of the Sale Event; and
• in the case of a Qualifying Transaction, the price per Share set for the underlying securities that are to be issued as part of the Qualifying Transaction
• of which the Noteholder has a conversion discount of
F-79
|
Gelteq Limited Note 23. Borrowings (cont.) |
|
The convertible note balance as at 30 June, 2023 comprises of convertible note funds received $
Since the year ended June 30, 2023, the Company has issued the following additional convertible notes (on the same terms and conditions as the previous convertible notes);
• September 2023, $
• October 2023, $
The total amount raised from the convertible note issue was $
On 2 February 2024, the Board of Directors approved the issuance of convertible notes (the “February 2024 Convertible Note”) to raise up to AUD$
On 27 May 2024, the Board of Directors approved the issuance of convertible notes (the “May 2024 Convertible Note”) to raise up to AUD$
Each holder of Convertible Note may, on a Liquidity event, or at least 90 days prior to Maturity, may elect to either Convert their Notes or redeem for Australian cash repayment. If the Noteholder elects to Convert, the number of fully paid ordinary shares to be issued in satisfaction of the Convertible Notes will be determined by the market value being, determined as;
• in the case of a Listing, the price per Share set for the underlying securities that are offered for issue as part of the Listing;
• in the case of a Sale Event, the price per Share set for the underlying securities that are to be sold as part of the Sale Event; and
• in the case of a Qualifying Transaction, the price per Share set for the underlying securities that are to be issued as part of the Qualifying Transaction of which the Noteholder has a conversion discount of
The table below shows the movement of Convertible Notes during the respective periods.
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Opening convertible note balance |
|
|
|||
|
Convertible notes issued – received in cash |
|
|
|
||
|
Convertible notes issued – accrued (owing) |
( |
) |
|
||
|
Interest accrued |
|
|
|
||
| |
|
|
|||
There was repayment of interest or loans/convertible notes during the period ended 30 June 2024 (30 June 2023: ).
Refer to note 33 for further information on related parties.
F-80
|
Gelteq Limited |
|
Note 24. Lease liabilities
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current liabilities |
||||
|
Lease liability |
|
|||
Refer to note 28 for further information on financial instruments.
Note 25. Employee benefits provisions
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Current liabilities |
||||
|
Provision for Annual leave |
| | ||
|
Non-current liabilities |
||||
|
Long service leave |
|
|||
| |
|
|||
Amounts not expected to be settled within the next 12 months
In 2024, the current provision for employee benefits includes all unconditional entitlements where employees have completed the required period of service and also those where employees are entitled to pro-rata payments in certain circumstances. The entire amount is presented as current, since the Consolidated Entity does not have an unconditional right to defer settlement. However, based on past experience, the Consolidated Entity does not expect all employees to take the full amount of accrued leave or require payment within the next 12 months.
Employee entitlements:
Annual leave
|
Consolidated |
||||||
|
30 June |
30 June |
|||||
|
$ |
$ |
|||||
|
Opening Balance |
|
|
|
|
||
|
Annual leave taken |
( |
) |
( |
) |
||
|
Additional provisions raised |
|
|
|
|
||
|
Closing balance |
|
|
|
|
||
Long Service leave
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Opening balance |
||||
|
Long service leave taken during the period |
||||
|
Additional provisions raised |
|
|||
|
Closing balance |
|
|||
F-81
|
Gelteq Limited |
|
Note 26. Issued capital
|
Consolidated |
||||||||
|
30 June |
30 June |
30 June |
30 June |
|||||
|
Shares |
Shares |
$ |
$ |
|||||
|
Ordinary shares – fully paid |
|
|
|
|
||||
Movements in ordinary share capital
|
Details |
Date |
Shares |
Issue price |
$ |
||||||
|
Opening balance |
1 July 2022 |
|
|
|
|
|||||
|
Shares issued to existing shareholders |
26 September 2022 |
|
$ |
|
|
|
||||
|
Share capital raising costs |
|
( |
) |
|||||||
|
Opening balance |
30 June 2023 |
|
|
|
|
|||||
|
Closing balance |
30 June 2024 |
|
|
|
|
|||||
On 26 September 2022, the Company issued
Ordinary shares
Ordinary shares entitle the holder to participate in dividends and the proceeds on the winding up of the Company in proportion to the number of and amounts paid on the shares held. The fully paid ordinary shares have no par value and the Company does not have a limited amount of authorised capital.
On a show of hands every member present at a meeting in person or by proxy shall have
Capital risk management
The Consolidated Entity’s objectives when managing capital is to safeguard its ability to continue as a going concern, so that it can provide returns for shareholders and benefits for other stakeholders and to maintain an optimum capital structure to reduce the cost of capital.
Capital is regarded as total equity, as recognised in the statement of financial position, plus net debt. Net debt is calculated as total borrowings less cash and cash equivalents. The Consolidated Entity may issue shares to investors and suppliers (and employees) time to time to raise capital and compensate for services received.
In order to maintain or adjust the capital structure, the Consolidated Entity may adjust the amount of dividends paid to shareholders, return capital to shareholders, issue new shares or sell assets to reduce debt.
Note 27. Dividends
There were no dividends paid, recommended or declared during the current or previous financial year.
Note 28. Financial risk management
Financial risk management objectives
The consolidated entity’s activities expose it to a variety of financial risks: market risk (including foreign currency risk, price risk and interest rate risk), credit risk and liquidity risk.
The consolidated entity’s overall risk management program focuses on the unpredictability of financial markets and seeks to minimise potential adverse effects on the financial performance of the consolidated entity.
F-82
|
Gelteq Limited Note 28. Financial risk management (cont.) |
|
The consolidated entity uses different methods to measure different types of risk to which it is exposed. These methods include sensitivity analysis in the case of interest rate, foreign exchange and other price risks, ageing analysis for credit risk and beta analysis in respect of investment portfolios to determine market risk.
Risk management is carried out by senior finance executives (‘finance’) under policies approved by the Board of Directors (‘the Board’). These policies include identification and analysis of the risk exposure of the Consolidated Entity and appropriate procedures, controls and risk limits. Finance identifies, evaluates and hedges financial risks within the Consolidated Entity’s operating units. Finance reports to the Board on a monthly basis.
Market risk
Foreign currency risk
The Consolidated Entity is not currently exposed to significant foreign currency risk. Foreign exchange risk arises from future commercial transactions and recognised financial assets and financial liabilities denominated in a currency that is not the entity’s functional currency. The risk is measured using sensitivity analysis and cash flow forecasting. Management understands, it will over the next twelve months, increase in dealing in foreign currencies and will have in place a risk management policy when it is required.
Price risk
The Consolidated Entity is not exposed to any significant price risk.
Cash flow and fair value interest rate risk
The Consolidated Entity has limited exposure to interest rate risks arising from long-term borrowings as these are based on fixed rates. There are no borrowings obtained at variable rates in the financial years to 30 June 2024 or 30 June 2023. All cash is held in chequing accounts or on hand, and do not earn interest.
Credit risk
The Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Consolidated Entity. The maximum exposure to credit risk at the reporting date to recognised financial assets is the carrying amount, net of any provisions for impairment of those assets, as disclosed in the statement of financial position and notes to the consolidated financial statements. The Consolidated Entity does not hold any collateral.
All trade and other receivables are current as at 30 June 2024 and 30 June 2023, with no balances past due.
The Consolidated Entity recorded no bad debt expense in the years ended 30 June 2024 or 30 June 2023. As of 30 June 2024 and 2023, there was no expected credit losses recorded.
Generally, trade receivables are written off when there is no reasonable expectation of recovery. Indicators of this include the failure of a debtor to engage in a repayment plan, no active enforcement activity and a failure to make contractual payments for a period greater than 1 year.
Liquidity risk
Vigilant liquidity risk management requires the Consolidated Entity to maintain sufficient liquid assets (mainly cash and cash equivalents) and available borrowing facilities to be able to pay debts as and when they become due and payable.
F-83
|
Gelteq Limited Note 28. Financial risk management (cont.) |
|
The Consolidated Entity manages liquidity risk by maintaining adequate cash reserves and available borrowing facilities by continuously monitoring actual and forecast cash flows and matching the maturity profiles of financial assets and liabilities. All loans as at 30 June 2024 are due to either directors, existing shareholders or related entities of the Consolidated Entity.
Liquidity risk — 2023
Since the year ended June 30, 2023, the Company has issued the following additional convertible notes (on the same terms and conditions as the previous convertible notes);
• September 2023, $
• October 2023, $
The $
Remaining contractual maturities
The following tables detail the Consolidated Entity’s remaining contractual maturity for its financial instrument liabilities. The tables have been drawn up based on the undiscounted cash flows of financial liabilities based on the earliest date on which the financial liabilities are required to be paid. The tables include both interest and principal cash flows disclosed as remaining contractual maturities and therefore these totals may differ from their carrying amount in the statement of financial position.
|
Consolidated – 30 June 2024 |
Weighted |
1 year or |
Between 1 and |
Between 2 and |
Remaining |
||||||
|
% |
$ |
$ |
$ |
$ |
|||||||
|
Non-derivatives |
|
||||||||||
|
Non-interest bearing |
|
||||||||||
|
Trade payables |
|
|
|
||||||||
|
Payroll liabilities |
|
|
|
||||||||
|
Other loans |
|
|
|
|
|||||||
|
|
|||||||||||
|
Interest-bearing – fixed rate |
|
||||||||||
|
Borrowings |
|
% |
|
|
|||||||
|
Borrowings – Shareholder Loans |
|
% |
|
|
|||||||
|
Borrowings – Convertible Notes |
|
% |
|
|
|||||||
|
Borrowings – Convertible Notes |
|
% |
|
|
|||||||
|
Total non-derivatives |
|
|
|
|
|||||||
F-84
|
Gelteq Limited Note 28. Financial risk management (cont.) |
|
|
Consolidated – 30 June 2023 |
Weighted |
1 year or |
Between 1 and |
Between 2 and |
Remaining |
||||||
|
% |
$ |
$ |
$ |
$ |
|||||||
|
Non-derivatives |
|
||||||||||
|
Non-interest bearing |
|
||||||||||
|
Trade and GST payables |
|
|
|
||||||||
|
Payroll liabilities |
|
|
|
||||||||
|
Other loans |
|
|
|
|
|||||||
|
|
|||||||||||
|
Interest-bearing – fixed rate |
|
||||||||||
|
Lease liability |
|
% |
|
|
|||||||
|
Borrowings |
|
% |
|
|
|||||||
|
Borrowings – Shareholder Loans* |
|
% |
|
|
|||||||
|
Borrowings – Convertible Notes |
|
% |
|
|
|||||||
|
Total non-derivatives |
|
|
|
|
|
||||||
____________
*
Fair Value
Fair Value Hierarchy
The following tables detail the Consolidated Entity’s assets and liabilities, measured or disclosed at fair value, using a three level hierarchy, based on the lowest level of input that is significant to the entire fair value measurement, being:
Level 1: Quoted prices (unadjusted) in active markets for identical assets or liabilities that the entity can access at measurement date
Level 2: Inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly or indirectly
Level 3: Unobservable inputs for the asset or liability
The Consolidated Entity had no assets or liabilities held at fair value using Level 3 inputs at 30 June 2024 (30 June 2023: No assets or liabilities held at fair value).
Note 29. Key management personnel
Key management personnel (KMP) are those persons having authority and responsibility for planning, directing and controlling the activities of the Consolidated Entity, including the directors of the company as listed on page F-6 immediately above Note 2, and the Financial Controller of the company. There is a pro-rata allocation of compensation for the time at the office for any KMP which have joined or left the Consolidated Entity during the reporting year.
F-85
|
Gelteq Limited Note 29. Key management personnel (cont.) |
|
Directors
The following persons were directors of Gelteq Limited during the financial year and the year ended 30 June 2023:
|
Mr. Simon Hayden Szewach |
(Executive Chairman) |
|
|
Mr. Nathan Jacob Givoni |
(Executive Director) |
|
|
Mr. Jeffrey W. Olyniec |
(Non-Executive Director) |
|
|
Mr. Philip Dalidakis |
(Non-Executive Director) |
|
|
Prof David Morton |
(Non-Executive Director) — effective 28 February 2023 |
|
|
Mr. Paul Wynne |
(Non-Executive Director) — resigned 28 February 2023 |
Other key management personnel
The following person also had the authority and responsibility for planning, directing and controlling the major activities of the Consolidated Entity, directly or indirectly, during the financial year and the year ended 30 June 2023:
|
Mr. Neale Java |
(Chief Financial Officer) — resigned 14 February 2023 |
|
|
Mr. Craig Young |
(Chief Financial Officer) — effective 20 February 2023; resigned on 14 January 2024 |
The aggregate compensation paid/payable to members of key management personnel of the Consolidated Entity is set out below:
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Short-term employee benefits |
|
|
|
||
|
Post-employment benefits |
|
|
|
||
|
Share-based payments |
( |
) |
|||
| | |
|
|||
Some of the above amounts were paid to related management entities
Note 30. Contingent assets & Liabilities and Commitments
Contingent assets & Liabilities and Commitments — 2024
On 13 February 2024, the Company entered into a consulting contract with ARC Group Limited. for advice in connection with the Initial Public Offering (IPO) in return for a cash payment of US$
Contingent assets & Liabilities and Commitments — 2023
On 25 April, 2023, the Company entered into a new underwriting agreement with R.F Lafferty & Co, Inc, which ended on 25 October, 2023, and has the following terms and conditions;
i. Raise up to USD$
ii.
iii.
F-86
|
Gelteq Limited Note 30. Contingent assets & Liabilities and Commitments (cont.) |
|
iv. USD$
v. Legal fees up to USD$
vi. Share purchase warrants of
The Company paid the upfront advance of USD$
Subsequent to the reporting period, on 17 November, 2023, the letter of engagement was further extended until 31 January, 2024 on the above terms.
There were no other contingent assets, contingent liabilities and commitments as at 30 June 2023.
Note 31. Capital commitments — Property, plant and equipment
The Consolidated Entity had capital commitments as at 30 June 2024 and 30 June 2023.
Note 32. Lease commitments
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Lease commitments – operating |
||||
|
Committed at the reporting date: |
||||
|
Within one year |
|
|||
|
One to five years |
||||
| |
||||
Note 33. Related party transactions
Parent entity
Gelteq Limited is the parent entity.
Subsidiaries
Interests in subsidiaries are set out in note 34.
Key management personnel
Disclosures relating to key management personnel are set out in note 29.
F-87
|
Gelteq Limited Note 33. Related party transactions (cont.) |
|
Transactions with related parties
The following transactions occurred with related parties:
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Payment for other expenses: |
|
||||
|
Interest expense on loans from directors (as part of shareholder loan and convertible note issue)* |
|
|
|
||
|
Interest expense on loans from controlling entity* |
|
|
|
||
|
Management and consulting services** |
|
|
|
||
|
|
|||||
|
Other transactions: |
|
||||
|
Gain on modification of loans |
( |
) |
|||
____________
*
**
Outstanding balances arising from transactions with related parties:
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Receivables from related parties |
||||
|
Prepayment* |
|
|
||
|
Trade receivables** |
|
|
||
|
Total Receivables from related parties |
|
|
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Payables to related parties |
||||
|
Payables to by key management personnel directly*** |
|
|
____________
*
**
***
F-88
|
Gelteq Limited Note 33. Related party transactions (cont.) |
|
Loans to/from related parties
The following balances are outstanding at the reporting date in relation to loans with related parties:
Loans from related parties (2024)/Directors (2023)
|
Consolidated |
|||||
|
30 June |
30 June |
||||
|
$ |
$ |
||||
|
Beginning of the period* |
|
|
|
||
|
Reclassify >5% holder loan as related party loan(i) |
|
|
|||
|
Modification of fair value on extinguishment |
( |
) |
|||
|
Interest accrued prior to modification |
|
|
|||
|
Interest accrued post modification |
|
|
|
||
|
Amounts received during the period |
|
||||
|
Closing Balance |
|
|
|
||
____________
*
Subsequent to 30 June, 2023, the loans were extended with a new maturity date of
(i)
Loans from associated entities
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
$ |
$ |
|||
|
Opening balance |
|
|
||
|
Interest charged |
|
|
||
| |
|
|||
F-89
|
Gelteq Limited Note 33. Related party transactions (cont.) |
|
Convertible notes from Related Parties (2024)/Directors* (2023)
|
Consolidated |
||||
|
30 June |
30 June |
|||
|
AUD$ |
AUD$ |
|||
|
Opening Balance |
|
|||
|
Reclassify >5% holder convertible note as related party loan(i) |
|
|||
|
Proceeds from convertible note issue |
|
|
||
|
Interest accrued |
|
|
||
|
Closing Balance |
|
|
||
____________
* The Convertible Notes from directors relates to:
– for 2023, convertible notes received from Jeffrey Olyniec, Non — Executive Director; and
– for 2024, convertible notes received from an entity related to Nathan Givoni, Executive Director, and Jeffrey Olyniec, Non-Executive Director.
(i)
Terms and conditions
Transactions with related parties have not undergone a formal benchmarking process to establish whether arrangements are conducted under normal market terms and conditions, accordingly, such transactions may not be considered at arm’s length. Related party loans are either unsecured, interest-free and payable on demand or are subject to unsecured loan agreements with fixed terms and interest payable.
Interest-free loans are noted accordingly.
No adjustment has been made to their carrying value. The parent company has not provided any guarantees in relation to any debts incurred by its subsidiaries.
Other related party transactions
On 30 October 2021, the Company entered into a lease agreement with the Lifestyle Breakthrough Holdings U/T to rent office space and incurred rental expense of $
Note 34. Interests in subsidiaries
(a) Information about principal subsidiaries
The subsidiaries listed below have share capital consisting solely of ordinary shares, which are held directly by the Consolidated Entity. The proportion of ownership interests held equals the voting rights held by the Consolidated Entity.
F-90
|
Gelteq Limited Note 34. Interests in subsidiaries (cont.) |
|
| Name | Principal place of business/Country | Ownership interest | ||||||
| 30 June | 30 June | |||||||
| % | % | |||||||
| Nutrigel Unit Trust | | | % | | % | |||
| Nutrigel Pty Ltd | | | % | | % | |||
| Sport Supplements Unit Trust | | | % | | % | |||
| Sport Supplements Pty Ltd | | | % | | % | |||
(b) Significant Restrictions
There are no significant restrictions over the Consolidated Entity’s ability to access or use assets, and settle liabilities, of the Consolidated Entity.
Note 35. Remuneration of auditors
During the financial year the following fees were paid or payable for services provided by the auditors of the Company:
|
30 June |
30 June |
|||
|
Audit or review of the consolidated financial statements – UHY Haines Norton |
|
|
||
|
Audit and Review Services – M&K CPAS, PLLC |
|
|||
| |
|
____________
* Audit fees paid or payable to UHY Haines Norton are included as part of our IPO expenses.
On May 21, 2024, UHY Haines Norton resigned solely as our independent auditor for PCAOB audits, and on May 21, 2024, we appointed M&K CPAS, PLLC as our new independent auditor. However, UHY Haines Norton will remain as our independent auditor for Australian reporting purposes
Note 36. Events after the reporting period
Events after the reporting period — 2024
Subsequent to 30 June 2024, loans from shareholders, which appear in the Consolidated Entity’s Statement of Financial Position as at 30 June 2024 as current liabilities with a balance of $
The Company completed its Initial Public Offering (IPO) on 30 October 2024, issuing
The Company was listed on NASDAQ on 29 October 2024.
No other matter or circumstance has arisen since 30 June 2024 that has significantly affected, or may significantly affect the Consolidated Entity’s operations, the results of those operations, or the Consolidated Entity’s state of affairs in future financial years.
Events after the reporting period — 2023
In the year after 30 June 2023, the Company has continued with work on its Initial Public Offer (IPO) and the proposed listing of its securities on the Nasdaq Capital Market (NASDAQ). On 3 August 2023, the Company lodged an updated filing with the U.S. Securities and Exchange Commission (SEC) and NASDAQ, the Prospectus for its proposed IPO. This Prospectus is currently being updated with 30 June, 2023 financials to be resubmitted to the SEC.
F-91
|
Gelteq Limited Note 36. Events after the reporting period (cont.) |
|
Since the year ended June 30, 2023 the Company has issued the following additional convertible notes (on the same terms and conditions as the previous convertible notes);
• September 2023, $
• October 2023, $
The $
During October 2023, all existing shareholder loan holders agreed to extend their loans, on the same terms and conditions, with a new maturity date of
On September 29, 2023, the Board, via a circular resolution, accepted the resignation of Suzanne Irwin as Company Secretary and appointed Craig Young as the new Company Secretary.
No other matter or circumstance has arisen since 30 June 2023 that has significantly affected, or may significantly affect the Consolidated Entity’s operations, the results of those operations, or the Consolidated Entity’s state of affairs in future financial years.
Note 37. Reconciliation of loss before income tax to net cash used in operating activities
|
Consolidated |
||||||
|
30 June |
30 June |
|||||
|
$ |
$ |
|||||
|
Loss before income tax expense for the year |
( |
) |
( |
) |
||
|
Adjustments for: |
— |
|
— |
|
||
|
Depreciation and amortisation |
|
|
|
|
||
|
Gain on loan modification |
|
( |
) |
|||
|
Foreign exchange differences |
|
( |
) |
|||
|
Interest expense |
|
|
|
|
||
|
— |
|
— |
|
|||
|
Change in operating and assets and liabilities: |
— |
|
— |
|
||
|
(Increase) in other debtors – research & development refund |
|
( |
) |
|||
|
(Increase)/decrease in GST receivable |
( |
) |
|
|
||
|
Increase/(decrease) in GST payable |
( |
) |
|
|
||
|
(Increase) in accounts receivables |
|
|
( |
) |
||
|
(Increase)/decrease in inventory |
|
|
|
|||
|
(Increase)/decrease in prepayments and other assets |
|
|
|
|
||
|
Increase/(decrease) in deferred revenue |
|
|
( |
) |
||
|
Increase in payroll liabilities |
|
|
|
|
||
|
Increase in provision for employee leave |
|
|
|
|
||
|
Increase in trade payables and accruals |
( |
) |
|
|
||
|
Net cash used in operating activities |
( |
) |
( |
) |
||
|
Reconciliation of cash |
30 June |
30 June |
||
|
$ |
$ |
|||
|
Cash on hand |
|
|||
|
Cash at Bank – Gelteq |
|
|
||
|
Cash at Bank – Nutrigel Unit Trust |
|
|||
|
Cash at Bank – Sport Supplements Pty Ltd |
||||
| |
|
F-92
|
Gelteq Limited |
|
Note 38. Changes in liabilities arising from financing activities
|
Consolidated |
Interest |
Lease |
Total |
||||||
|
$ |
$ |
$ |
|||||||
|
Balance at 1 July 2022 |
|
|
|
|
|
|
|||
|
Net cash from financing activities |
|
|
( |
) |
|
* |
|||
|
Gain on loan modification |
( |
) |
|
( |
) |
||||
|
Other changes – accrued interest and convertible notes (note 23) |
|
|
|
|
|
||||
|
Balance at 30 June 2023 |
|
|
|
|
|
|
|||
|
Balance at 1 July 2023 |
|
|
|
|
|
|
|||
|
Net cash from/(used in) financing activities |
|
|
( |
) |
|
|
|||
|
Accrued interest |
|
|
|
|
|
||||
|
Other changes |
( |
) |
|
( |
) |
||||
|
Balance at 30 June 2024 |
|
|
|
|
|
||||
____________
*
Note 39. Loss per share
|
Consolidated |
||||||
|
30 June |
30 June |
|||||
|
$ |
$ |
|||||
|
Loss after income tax attributable to the owners of Gelteq Limited |
( |
) |
( |
) |
||
|
Number |
Number |
|||
|
Weighted average number of ordinary shares used in calculating basic earnings per share |
|
|
||
|
Weighted average number of ordinary shares used in calculating diluted earnings per share* |
|
|
|
$ |
$ |
|||||
|
Basic loss per share |
( |
) |
( |
) |
||
|
Diluted loss per share |
( |
) |
( |
) |
||
____________
*
Share capital subscribed and to be issued is included within earnings per share calculations per IAS 33. Shares are usually included in the weighted average number of shares from the date consideration is receivable (which is generally the date of their issue). Therefore ordinary shares issued in exchange for cash are included when cash is receivable and ordinary shares issued for the rendering of services to the entity are included as the services are rendered.
On 26 September 2022, the Company issued
F-93
|
Gelteq Limited Note 39. Loss per share (cont.) |
|
Refer to Note 26 for further information.
On March 24, 2022, the Company entered into a consulting contract with a counterparty pursuant to which the counterparty will advise in connection with the initial public offering in return for a monthly retainer of a fixed dollar amount with additional fixed cash payments to be made upon the satisfaction of certain conditions and
The directors received Board approval on May 5, 2023 to issue up to AUD$
Note 40. Share-based payments
On 14 February, 2023, Mr. Neale Java resigned as Chief Financial Officer of the company and as a result he forfeited his rights to ordinary shares in the company. The A$
F-94
|
Gelteq Limited |
|
In accordance with a resolution of the directors of Gelteq Limited, the directors of the Company declare that:
In the directors’ opinion:
• the consolidated financial statements and notes for the two years ended 30 June 2024 set out in this document are in accordance with requirements of the International Financial Reporting Standards (IFRS), including:
(i) complying with Accounting Standards, as issued by the International Accounting Standards Board, and
(ii) present fairly in all material respects the Consolidated Entity’s financial position as at 30 June 2024 and 30 June 2023, and the results of its operations and its cash flows for each of the years ended 30 June 2024 and 30 June 2023, and
• there are reasonable grounds to believe that the Consolidated Entity will be able to pay its debts as and when they become due and payable.
|
On behalf of the directors |
||||
|
/s/ Simon H. Szewach |
||||
|
Simon H. Szewach |
||||
|
Executive Chairman |
||||
|
15 November 2024 |
F-95
Gelteq Limited
4,000,000 Ordinary Shares
________________________________
PROSPECTUS
, 2025
________________________________
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers.
Australian law
Australian law provides that a company or a related body corporate of the company may provide for indemnification of a personas an officer or auditor of the company, except to the extent of any of the following liabilities incurred as an officer or auditor of the company:
• a liability owed to the company or a related body corporate of the company;
• a liability for a pecuniary penalty order made under section 1317G or a compensation order under section 961M, 1317H, 1317HA,1317HB, 1317HC or 1317HE of the Corporations Act; or
• a liability that is owed to someone other than the company or a related body corporate of the company and did not arise out of conduct in good faith.
Australian law provides that a company or related body corporate of the company must not indemnify a person against legal costs incurred in defending an action for a liability incurred as an officer or auditor of the company if the costs are incurred:
• in defending or resisting proceedings in which the officer or director is found to have a liability for which they cannot be indemnified as setout above;
• in defending or resisting criminal proceedings in which the person is found guilty;
• in defending or resisting proceedings brought by the ASIC or a liquidator for a court order if the grounds for making the order are found by the court to have been established (except costs incurred in responding to actions taken by the ASIC or a liquidator as part of an investigation before commencing proceedings for the court order); or
• in connection with proceedings for relief to the officer or a director under the Corporations Act, in which the court denies the relief.
Constitution. We were incorporated as a proprietary company limited by shares under the laws of Australia in October 2018. The name of the Company was changed from Myhypo Pty Ltd to Gelteq Pty Ltd in connection with the expansion of the business across a wider set of markets and became Gelteq Limited upon conversion into a public company on May 26, 2022. Our Constitution provide that, to the extent permitted by and subject to any applicable law, for the indemnification of each director, secretary and officer of our company, or a subsidiary of our company against any liability incurred by that person in such capacity, and for any legal costs incurred in defending or resisting (or otherwise in connection with) proceedings, whether civil or criminal or of an administrative or investigatory nature, in which the person becomes involved because of that capacity.
SEC Position. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling our company pursuant to the foregoing provisions, our company has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Item 7. Recent sales of unregistered securities.
During the prior three years, we issued and sold to third parties the securities listed below without registering the securities under the Securities Act of 1933, as amended (the “Securities Act”) pursuant to Section 4(a)(2) thereof and Regulations D and S thereunder. None of these transactions involved any public offering. All our securities were
II-1
sold through private placement either (i) outside the United States or (ii) in the United States to a limited number of investors in transactions not involving any public offering. As discussed below, we believe that each issuance of these securities was exempt from, or not subject to, registration under the Securities Act.
• On May 5, 2023, our board of directors approved the issuance of convertible notes (the “May 2023 Convertible Note”) to raise up to AUD$1,000,000. Each May 2023 Convertible Note shall have a face value of AUD$1, an annual interest rate of 12% and have a maturity date of December 31, 2025. Each holder of a May 2023 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms of the May 2023 Convertible Note, either elect to convert their May 2023 Convertible Note into Ordinary Shares or redeem their May 2023 Convertible Note for an Australian cash payment. On October 3, 2023, we have closed the May 2023 Convertible Note offering raising approximately AUD$1,004,889 (AUD$410,000 plus USD$400,000 calculated at the daily exchange rate when each amount was received).
• On February 2, 2024, our board of directors approved the issuance of convertible notes (the “February 2024 Convertible Note”) to raise up to AUD$400,000. Each February 2024 Convertible Note shall have a face value of AUD$1, an annual interest rate of 6% and have a maturity date of December 31, 2025. Each holder of a February 2024 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms therein, either elect to convert their February 2024 Convertible Note into Ordinary Shares at a conversion discount rate of 22% or redeem their February 2024 Convertible Note for an Australian cash payment. The Company received approximately AUD$357,338 (approximately AUD$75,000 plus approximately USD$185,000 calculated at the daily exchange rate when each amount was received) through the issuance of February 2024 Convertible Notes and it was closed on March 26. 2024.
• On May 27, 2024, our board of directors approved the issuance of convertible notes (the “May 2024 Convertible Note”) to raise up to AUD$1,000,000. Each May 2024 Convertible Note had a face value of AUD$1, an annual interest rate of 6% and have a maturity date of December 31, 2025. Each holder of a May 2024 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms therein, either elect to convert their May 2024 Convertible Note into Ordinary Shares at a conversion discount rate of 22% or redeem their May 2024 Convertible Note for an Australian cash payment. As of the date of this prospectus, the Company has received approximately AUD$1million (approximately AUD$315,000 plus approximately USD$450,000 calculated at the daily exchange rate when each amount was received) through the issuance of the May 2024 Convertible Notes.
To reduce the Company’s debt position and improve its balance sheet, the Company in January 2025 offered existing convertible note and shareholder loan holders the ability to convert their loans into equity, be repaid or continue to maturity. For the then outstanding convertible notes, a total of AUD $822,184 (approximately USD$534,420) was converted in March 2025 by the election of the noteholders into Ordinary Shares at a share price of USD$2.14. In March 2025, the Company paid to loan holders an aggregate of AUD$772,136 (approximately USD$501,888) in order to redeem their loans. The remaining principal and interest on the outstanding shareholder loans will accrue until maturity in December 2025.
On February 21, 2025, our board of directors approved the issuance of convertible notes (the “February 2025 Convertible Note”) to raise up to AUD$1,500,000. Each February 2025 Convertible Note had a face value of AUD$1, an annual interest rate of 20% and have a maturity date of July 1, 2026. Each holder of a February 2025 Convertible Note may at any time elect to convert their February 2025 Convertible Note into Ordinary Shares at a conversion price of USD$2.00. Each holder of a February 2025 Convertible Note may, prior to 90 days of their maturity date and pursuant to the terms therein, either elect to convert their February 2025 Convertible Note into Ordinary Shares at a conversion price of USD$2.00 or redeem their February 2025 Convertible Note for an Australian cash payment. As of the date of this prospectus, the Company has received approximately AUD$580,000 (approximately USD$377,000) through the issuance of the February 2025 Convertible Notes.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. Unless otherwise specified above, we believe these transactions were exempt from registration under the Securities Act in reliance on (i) Section 4(a)(2) of the Securities Act(and Regulation D promulgated thereunder), (ii) Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or under benefit plans and contracts relating to compensation as provided under Rule 701 or (iii) Regulation S promulgated under the Securities Act as transactions not made to persons in the United States with no directed selling efforts made in the United States. The recipients of the securities in each of these transactions represented
II-2
their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed on the share certificates issued in these transactions. All recipients had adequate access, through their relationships with us, to information about us. The sales of these securities were made without any general solicitation or advertising.
The latest transaction described below involved a placement agent, placement agent discount and commission,
• On September 26 2022, the Company issued 746,268 fully paid Ordinary Shares to certain Australian investors who participated in the Pre-IPO Raise at an issue price of USD$1.34 per share.
The Company closed its initial public offering on October 30, 2024, issuing 1.3 million ordinary shares at an issue price of US$4.00 per share and raising USD$5.2 million (approximately AUD$8.00 million) before deducting underwriting discounts and offering expenses.
Item 8. Exhibits and Financial Statement Schedules
(a) The following documents are filed as part of this registration statement:
EXHIBIT INDEX
The following documents are filed as part of this registration statement:
|
|
|
|
|
To be |
||||||||||
|
Form |
File No. |
Exhibit |
Filing Date |
|||||||||||
|
1.1 |
F-1 |
333-280804 |
3.1 |
July 15, 2024 |
||||||||||
|
2.2 |
6-K |
001-42373 |
1.1 |
October 30, 2024 |
||||||||||
|
2.3 |
6-K |
001-42373 |
4.1 |
October 30, 2024 |
||||||||||
|
5.1 |
Opinion of Vistra Australia Legal Services Pty Ltd t/a Vistra Legal Australia |
X |
||||||||||||
|
10.1# |
F-1 |
333-280804 |
10.1 |
July 15, 2024 |
||||||||||
|
10.2# |
F-1 |
333-280804 |
10.2 |
July 15, 2024 |
||||||||||
|
10.3# |
F-1 |
333-280804 |
10.3 |
July 15, 2024 |
||||||||||
|
10.4# |
F-1 |
333-280804 |
10.4 |
July 15, 2024 |
||||||||||
|
10.5# |
Variation Agreement, dated May 15, 2021, by and among Monash University and MyHypo Pty Ltd |
F-1 |
333-280804 |
10.5 |
July 15, 2024 |
|||||||||
II-3
|
|
|
|
|
To be |
||||||||||
|
Form |
File No. |
Exhibit |
Filing Date |
|||||||||||
|
10.6# |
F-1 |
333-280804 |
10.8 |
July 15, 2024 |
||||||||||
|
10.7# |
F-1 |
333-280804 |
10.10 |
July 15, 2024 |
||||||||||
|
10.8 |
F-1 |
333-280804 |
10.11 |
July 15, 2024 |
||||||||||
|
10.9# |
Consulting Agreement, dated September 6, 2021, by and among Sosna & Co Inc. and Gelteq Pty Ltd |
F-1 |
333-280804 |
10.12 |
July 15, 2024 |
|||||||||
|
10.10# |
F-1 |
333-280804 |
10.14 |
July 15, 2024 |
||||||||||
|
10.11# |
F-1 |
333-280804 |
10.15 |
July 15, 2024 |
||||||||||
|
10.12#+ |
Executive Service Agreement, dated April 28, 2022, among Simon Hayden Szewach and Gelteq Pty Ltd |
F-1 |
333-280804 |
10.16 |
July 15, 2024 |
|||||||||
|
10.13#+ |
Executive Service Agreement, dated April 28, 2022, among Nathan Jacob Givoni and Gelteq Pty Ltd |
F-1 |
333-280804 |
10.17 |
July 15, 2024 |
|||||||||
II-4
|
|
|
|
|
To be |
||||||||||
|
Form |
File No. |
Exhibit |
Filing Date |
|||||||||||
|
10.14#+ |
F-1 |
333-280804 |
10.18 |
July 15, 2024 |
||||||||||
|
10.15# |
F-1 |
333-280804 |
10.19 |
July 15, 2024 |
||||||||||
|
10.16# |
F-1 |
333-280804 |
10.20 |
July 15, 2024 |
||||||||||
|
10.17# |
F-1 |
333-280804 |
10.21 |
July 15, 2024 |
||||||||||
|
10.18# |
F-1 |
333-280804 |
10.22 |
July 15, 2024 |
||||||||||
II-5
|
|
|
|
|
To be |
||||||||||
|
Form |
File No. |
Exhibit |
Filing Date |
|||||||||||
|
10.19# |
F-1 |
333-280804 |
10.23 |
July 15, 2024 |
||||||||||
|
10.20# |
Consulting Agreement, dated February 13, 2024, by and among Arc Group Limited and Gelteq Ltd. |
F-1 |
333-280804 |
10.24 |
July 15, 2024 |
|||||||||
|
10.21# |
F-1/A |
333-280804 |
10.25 |
September 12, 2024 |
||||||||||
|
10.22 |
F-1/A |
333-280804 |
10.26 |
September 12, 2024 |
||||||||||
|
10.23# |
Statement of Work, dated December 2, 2024, by and between WIPC Marketing Limited and Gelteq Limited |
X |
||||||||||||
|
10.24# |
X |
|||||||||||||
|
10.25# |
Executive Service Agreement, dated October 4, 2024, among Dr. Paul Wynne and Gelteq Limited |
X |
||||||||||||
|
10.26 |
6-K |
001-42373 |
10.1 |
March 14, 2025 |
||||||||||
|
10.27 |
6-K |
001-42373 |
10.2 |
March 14, 2025 |
||||||||||
|
10.28 |
X |
|||||||||||||
II-6
|
|
|
|
|
To be |
||||||||||
|
Form |
File No. |
Exhibit |
Filing Date |
|||||||||||
|
21.1 |
F-1 |
333-280804 |
21.1 |
July 15, 2024 |
||||||||||
|
23.1 |
Consent of Vistra Australia Legal Services Pty Ltd t/a Vistra Legal Australia (see Exhibit 5.1) |
X |
||||||||||||
|
23.2 |
Consent of M&K CPAS PLLC on consolidated financial statements for the year ended June 30, 2024 |
X |
||||||||||||
|
23.3 |
Consent of UHY Norton Haines on consolidated financial statements for the year ended June 30, 2023 |
X |
||||||||||||
|
11.1 |
F-1 |
333-280804 |
14.1 |
July 15, |
||||||||||
|
11.2 |
20-F |
001-42373 |
11.2 |
November 15, 2024 |
||||||||||
|
97 |
20-F |
001-42373 |
2.1 |
November 15, 2024 |
||||||||||
|
104 |
X |
|||||||||||||
____________
# Pursuant to Item 601(b)(10)(iv) of Regulation S-K, certain identified information marked with [*****] has been excluded from the exhibit because it is both (i) not material and (ii) the type that the registrant treats as private or confidential.
+ Indicates a management contract or compensatory plan.
# Portions of the exhibit have been omitted as the registrant has determined that: (i) the omitted information is not material; and (ii) the omitted information is the type that the registrant treats as private or confidential.
Item 9. Undertakings.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the provisions described in Item 6, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
i. To include any prospectus required by Section 10(a)(3) of the Securities Act;
ii. To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective Registration Statement;
II-7
iii. To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(5) That, for the purpose of determining liability under the Securities Act to any purchaser:
Each prospectus filed by the Registrant pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the small business issuer pursuant to the foregoing provisions, or otherwise, the small business issuer has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the small business issuer of expenses incurred or paid by a director, officer or controlling person of the small business issuer in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the small business issuer will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Melbourne, Australia, on June 30, 2025.
|
Signature |
Title |
Date |
||
|
/s/ Nathan J. Givoni |
Chief Executive Officer |
June 30, 2025 |
||
|
Name: Nathan J. Givoni |
POWER OF ATTORNEY
KNOW ALL MEN BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Nathan J. Givoni his true and lawful attorney-in-fact, with full power of substitution and re-substitution for him and in his name, place and stead, in any and all capacities to sign any and all amendments including post-effective amendments to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that said attorney-in-fact or his substitute, each acting alone, may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
|
Signature |
Title |
Date |
||
|
/s/ Nathan J. Givoni |
Chief Executive Officer and Director |
June 30, 2025 |
||
|
Name: Nathan J. Givoni |
(Principal Executive Officer) |
|||
|
/s/ Simon H. Szewach |
Chairman of the Board of Directors |
June 30, 2025 |
||
|
Name: Simon H. Szewach |
||||
|
/s/ Thuy-Linh Gigler |
Chief Financial Officer (Principal Financial |
June 30, 2025 |
||
|
Name: Thuy-Linh Gigler |
Officer and Principal Accounting Officer) |
|||
|
/s/ Jeffrey W. Olyniec |
Director |
June 30, 2025 |
||
|
Name: Jeffrey W. Olyniec |
||||
|
/s/ Philip A. Dalidakis |
Director |
June 30, 2025 |
||
|
Name: Philip A. Dalidakis |
II-9
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933 as amended, the undersigned, the duly authorized representative in the United States of America of Gelteq Limited has signed this registration statement or amendment thereto in Newark, Delaware on June 30, 2025.
|
Puglisi & Associates |
||||
|
By: |
/s/ Donald J. Puglisi |
|||
|
Name: |
Donald J. Puglisi |
|||
|
Title: |
Managing Director |
|||
II-10


